
Common name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
IUPAC name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
SMILES
C12[N](C(CC1)CC(C2)OC(=O)C(C)C)(C)C
Common name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
IUPAC name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
SMILES
C12[N](C(CC1)CC(C2)OC(=O)C(C)C)(C)C
INCHI
InChI=1S/C13H24NO2/c1-9(2)13(15)16-12-7-10-5-6-11(8-12)14(10,3)4/h9-12H,5-8H2,1-4H3/t10-,11+,12-
FORMULA
C13H24NO2

Common name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
IUPAC name
[(1S,5R)-8,8-dimethyl-8λ4-azabicyclo[3.2.1]octan-3-yl] 2-methylpropanoate
Molecular weight
226.335
clogP
-0.925
clogS
-2.477
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total Polar
Surface Area
26.3
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00387 | Anisotropine Methylbromide |
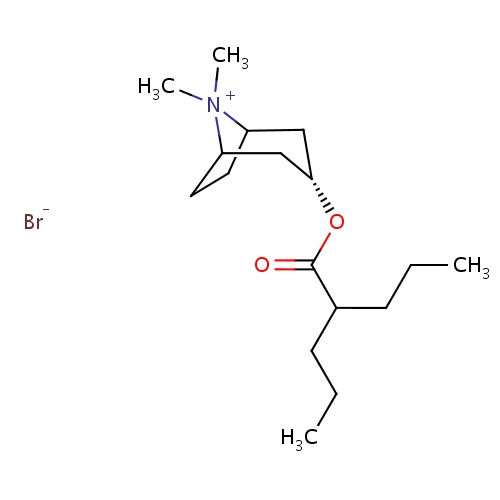
|
Muscarinic Antagonists; Cholinergic Antagonists; | For use in conjunction with antacids or histamine H. |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1i7z_ligand_frag_1.mol2 | 1i7z | 0.867925 | -7.16 | C1[C@H](C[C@H]2[N@@H+]([C@@H]1CC2)C)OC=O | 12 |
| 2pgz_ligand_frag_1.mol2 | 2pgz | 0.867925 | -7.02 | C1[C@@H]2[N@H+]([C@@H](CC2)C[C@@H]1OC=O)C | 12 |
| 1q72_ligand_frag_1.mol2 | 1q72 | 0.867925 | -6.48 | C1[C@@H]2[N@H+]([C@@H](CC2)C[C@@H]1OC=O)C | 12 |
| 2arm_ligand_1_0.mol2 | 2arm | 0.864407 | -6.16 | C[N@@H+]1[C@@]23CC[C@@]12C[C@H](C3)OC(=O)C | 13 |
| 1qb9_ligand_3_6.mol2 | 1qb9 | 0.8 | -6.90 | C1(CC[NH2+]CC1)O[C@@H]1CC[C@H]2[C@H]([N@@H+](C)[C@@H]3[C@@H]2CCCC3)C1 | 21 |
| 2iog_ligand_4_321.mol2 | 2iog | 0.763636 | -7.25 | C(O[C@@H]1C[C@@H]2[NH2+]C[C@H](C)[C@H]2CC1)C | 13 |
| 2iog_ligand_3_161.mol2 | 2iog | 0.763636 | -6.97 | C(O[C@@H]1C[C@@H]2[NH2+]CC[C@H]2CC1)C | 12 |
| 4ir6_ligand_2_5.mol2 | 4ir6 | 0.758065 | -7.22 | CC(=O)[C@H]1CC[C@@H]2C[C@@H](CC[N@@H+]12)OC1CCCCC1 | 19 |

