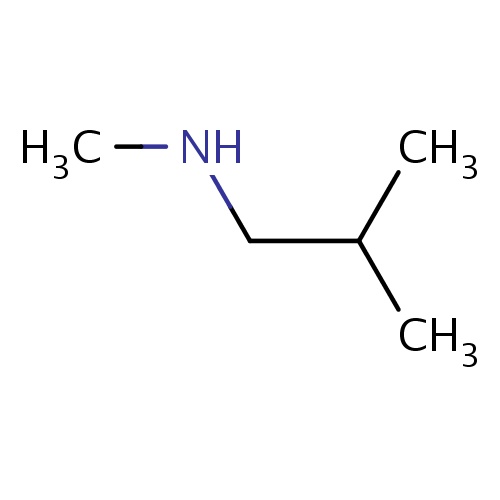
Common name
N,2-dimethylpropan-1-amine
IUPAC name
N,2-dimethylpropan-1-amine
SMILES
C(C(C)C)NC
Common name
N,2-dimethylpropan-1-amine
IUPAC name
N,2-dimethylpropan-1-amine
SMILES
C(C(C)C)NC
INCHI
InChI=1S/C5H13N/c1-5(2)4-6-3/h5-6H,4H2,1-3H3
FORMULA
C5H13N

Common name
N,2-dimethylpropan-1-amine
IUPAC name
N,2-dimethylpropan-1-amine
Molecular weight
87.163
clogP
0.511
clogS
-1.443
Frequency
0.0017
HBond Acceptor
0
HBond Donor
1
Total PolarSurface Area
12.03
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00397 | Lercanidipine |

|
Antihypertensive Agents; Calcium Channel Blockers; Cardiovascular System; Agents Acting on the Renin-Angiotensin System; Angiotensin II Antagonists and Calcium Channel Blockers; ACE Inhibitors and Calcium Channel Blockers; Dihydropyridine Derivatives; Selective Calcium Channel Blockers With Mainly Vascular Effects; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of Hypertension, management of angina pectoris and Raynaud's syndrome. |
| FDBD00566 | Amprenavir |
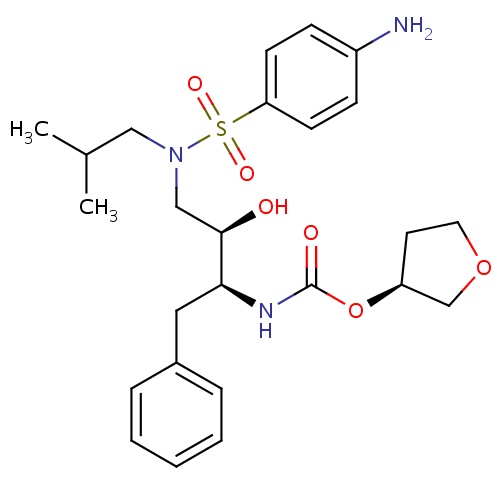
|
Anti-HIV Agents; Antibiotics, Antitubercular; Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of HIV-1 infection in combination with other antiretroviral agents. |
| FDBD01106 | Darunavir |
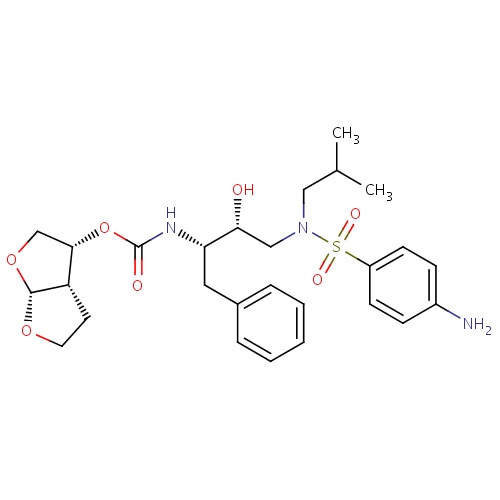
|
Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Darunavir, co-administered with ritonavir, and with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV) infection in antiretroviral treatment-experienced adult patients, such as those with HIV-1 strains resistant to more than one protease inhibitor. |
| FDBD01127 | Fosamprenavir |
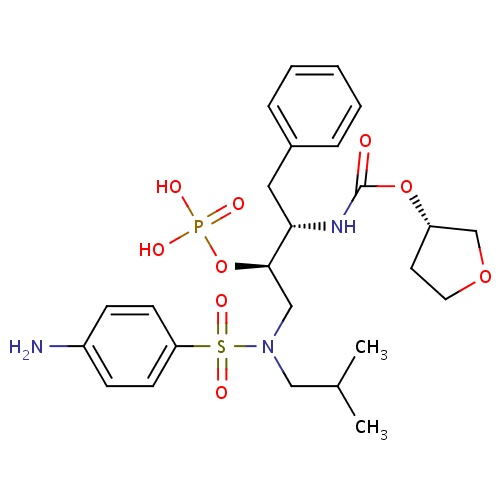
|
Anti-HIV Agents; Protease Inhibitors; HIV Protease Inhibitors; Prodrugs; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. The use of fosamprenavir is pending revision due to a potential association between the drug and myocardial infarction and dyslipidemia in HIV infected adults. |
| FDBD01728 | Technetium Tc-99m sestamibi |
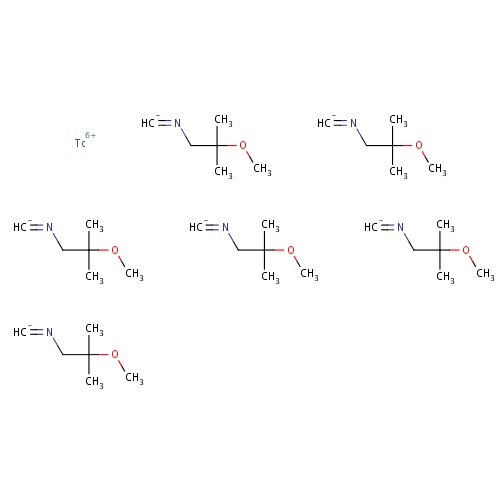
|
Diagnostic Radiopharmaceuticals; Cardiovascular System; Technetium (99Mtc) Compounds; Radiopharmaceuticals; |
5 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4bgk_ligand_3_3.mol2 | 4bgk | 1 | -6.34 | [N+](C)(C)(C)CCC | 7 |
| 4fmu_ligand_2_22.mol2 | 4fmu | 1 | -6.21 | C[NH2+]CCC | 5 |
| 1pot_ligand_3_10.mol2 | 1pot | 1 | -6.20 | C(C[NH2+]C)C | 5 |
| 3fhe_ligand_4_121.mol2 | 3fhe | 1 | -5.98 | C[NH+](CCC)C | 6 |
| 4fmu_ligand_3_22.mol2 | 4fmu | 1 | -5.98 | C([NH2+]C)CC | 5 |
| 1p0y_ligand_2_5.mol2 | 1p0y | 1 | -5.96 | C([NH2+]C)CC | 5 |
| 3k26_ligand_3_85.mol2 | 3k26 | 1 | -5.95 | C([N+](C)(C)C)CC | 7 |
| 3iiw_ligand_3_781.mol2 | 3iiw | 1 | -5.94 | C([N+](C)(C)C)CC | 7 |
| 3jzg_ligand_3_550.mol2 | 3jzg | 1 | -5.94 | CCC[N+](C)(C)C | 7 |
211 ,
22

