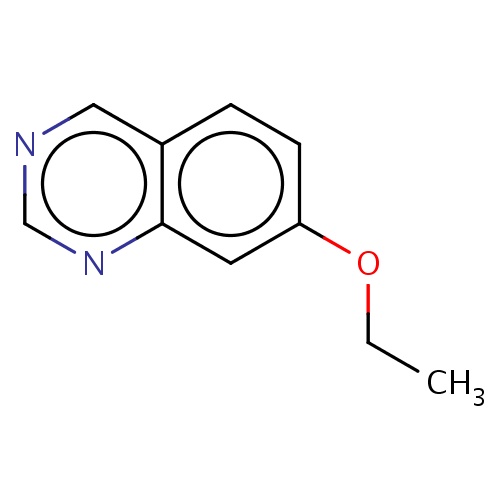
Common name
7-ethoxyquinazoline
IUPAC name
7-ethoxyquinazoline
SMILES
n1c2c(ccc(c2)OCC)cnc1
Common name
7-ethoxyquinazoline
IUPAC name
7-ethoxyquinazoline
SMILES
n1c2c(ccc(c2)OCC)cnc1
INCHI
InChI=1S/C10H10N2O/c1-2-13-9-4-3-8-6-11-7-12-10(8)5-9/h3-7H,2H2,1H3
FORMULA
C10H10N2O

Common name
7-ethoxyquinazoline
IUPAC name
7-ethoxyquinazoline
Molecular weight
174.199
clogP
2.248
clogS
-3.100
Frequency
0.0003
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
35.01
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00399 | Erlotinib |
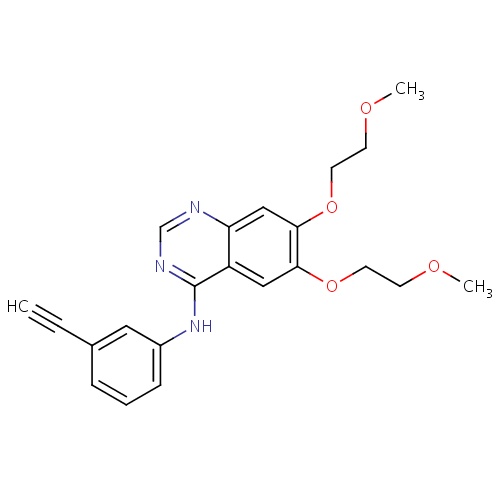
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Also for use, in combination with gemcitabine, as the first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4c2v_ligand_3_230.mol2 | 4c2v | 1 | -7.11 | C(Oc1cc2ncncc2cc1)C | 13 |
| 2vrx_ligand_3_64.mol2 | 2vrx | 1 | -7.06 | CCOc1cc2c(cc1)cncn2 | 13 |
| 2h8h_ligand_3_21.mol2 | 2h8h | 1 | -7.04 | C(Oc1ccc2cncnc2c1)C | 13 |
| 2vwu_ligand_3_46.mol2 | 2vwu | 1 | -7.00 | c1cc2cncnc2cc1OCC | 13 |
| 3mo5_ligand_3_310.mol2 | 3mo5 | 1 | -6.98 | n1cnc2c(c1)ccc(c2)OCC | 13 |
| 3mo2_ligand_3_214.mol2 | 3mo2 | 1 | -6.93 | n1cnc2c(c1)ccc(c2)OCC | 13 |
| 1kz8_ligand_2_27.mol2 | 1kz8 | 1 | -6.18 | C(C)Oc1ccc2cncnc2c1 | 13 |
101 ,
11

