
Common name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
IUPAC name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
SMILES
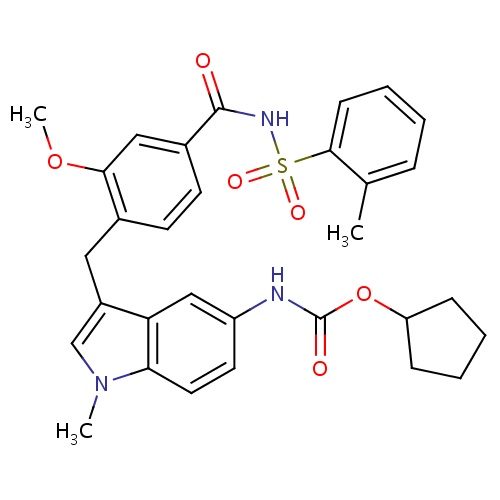
C1(CCCC1)OC(=O)Nc2cc3c(n(cc3C)C)cc2
Common name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
IUPAC name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
SMILES
C1(CCCC1)OC(=O)Nc2cc3c(n(cc3C)C)cc2
INCHI
InChI=1S/C16H20N2O2/c1-11-10-18(2)15-8-7-12(9-14(11)15)17-16(19)20-13-5-3-4-6-13/h7-10,13H,3-6H2,1-2H3,(H,17,19)
FORMULA
C16H20N2O2

Common name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
IUPAC name
cyclopentyl N-(1,3-dimethylindol-5-yl)carbamate
Molecular weight
281.414
clogP
1.570
clogS
-2.901
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total Polar
Surface Area
42.77
Number of Rings
3
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00416 | Zafirlukast |
|
Anti-Asthmatic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Leukotriene Receptor Antagonists; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | For the prophylaxis and chronic treatment of asthma. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1xdg_ligand_4_145.mol2 | 1xdg | 0.705882 | -5.54 | CN1[C@H](C[C@H](OC1=O)CC)C | 11 |
| 1xdg_ligand_3_90.mol2 | 1xdg | 0.705882 | -5.50 | N1[C@H](C[C@H](OC1=O)CC)C | 10 |
| 4lnb_ligand_2_0.mol2 | 4lnb | 0.666667 | -6.44 | O=C(OC(C)(C)C)N1CCC(CC1)C | 14 |
| 2pjt_ligand_2_26.mol2 | 2pjt | 0.666667 | -5.99 | CC1CCN(CC1)C(=O)OC(C)(C)C | 14 |
| 1xdg_ligand_3_65.mol2 | 1xdg | 0.661765 | -5.43 | CN1[C@H](C[C@H](OC1=O)C)C | 10 |
| 3elm_ligand_1_6.mol2 | 3elm | 0.650794 | -6.37 | C(C)(C)OC(=O)N1CCCCC1 | 12 |
| 4lnb_ligand_1_0.mol2 | 4lnb | 0.650794 | -6.30 | O=C(OC(C)(C)C)N1CCCCC1 | 13 |
| 2pjt_ligand_1_7.mol2 | 2pjt | 0.650794 | -5.74 | C1CCN(CC1)C(=O)OC(C)(C)C | 13 |
| 1xdg_ligand_2_25.mol2 | 1xdg | 0.647059 | -5.39 | N1[C@H](C[C@H](OC1=O)C)C | 9 |
| 4e4n_ligand_2_0.mol2 | 4e4n | 0.645161 | -7.10 | C1(CCCC1)NC(=O)OC(C)(C)C | 13 |
105 ,
11

