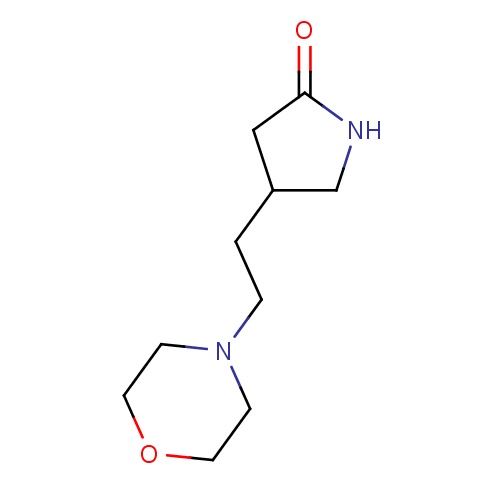
Common name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
IUPAC name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
SMILES
O=C1NCC(C1)CCN2CCOCC2
Common name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
IUPAC name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
SMILES
O=C1NCC(C1)CCN2CCOCC2
INCHI
InChI=1S/C10H18N2O2/c13-10-7-9(8-11-10)1-2-12-3-5-14-6-4-12/h9H,1-8H2,(H,11,13)/t9-/m0/s1
FORMULA
C10H18N2O2

Common name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
IUPAC name
(4S)-4-(2-morpholinoethyl)pyrrolidin-2-one
Molecular weight
198.262
clogP
1.244
clogS
-1.699
Frequency
0.0003
HBond Acceptor
3
HBond Donor
1
Total PolarSurface Area
41.57
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00428 | Doxapram |
|
Central Nervous System Stimulants; Respiratory System Agents; Respiratory System; Respiratory Stimulants; | For use as a temporary measure in hospitalized patients with acute respiratory insufficiency superimposed on chronic obstructive pulmonary disease. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4zim_ligand_1_0.mol2 | 4zim | 0.767857 | -7.89 | [C@H]1(CC[C@@H]2[C@H](C1)[C@H]1[C@H](CCCC1)[NH2+]2)C(=O)N1CCOCC1 | 21 |
| 4bsq_ligand_3_16.mol2 | 4bsq | 0.754386 | -7.01 | C(=O)(N1CCOCC1)[C@H]1[C@@H](CCC1)C(=O)NC | 17 |
| 4bsq_ligand_1_2.mol2 | 4bsq | 0.740741 | -6.51 | C(=O)(N1CCOCC1)C1CCCC1 | 13 |
| 1w2x_ligand_1_0.mol2 | 1w2x | 0.711864 | -6.50 | C1COCCN1C(=O)[C@H]1C[NH2+]CCC1 | 14 |
| 3ff6_ligand_1_0.mol2 | 3ff6 | 0.711864 | -6.23 | [C@@H]1(C[NH2+]CCC1)C(=O)N1CCOCC1 | 14 |
| 1biw_ligand_3_54.mol2 | 1biw | 0.690909 | -5.84 | C1(=O)N(CCCCC1)CCOC | 12 |
| 4hbm_ligand_2_5.mol2 | 4hbm | 0.68 | -5.48 | C(O)CN1CCCCC1=O | 10 |
| 4odf_ligand_4_71.mol2 | 4odf | 0.678571 | -6.63 | C1OC(C(=O)N(C1)[C@@H](C1CC1)C)(C)C | 14 |
| 4ogt_ligand_4_71.mol2 | 4ogt | 0.678571 | -6.53 | CC1(OCCN(C1=O)[C@H](C)C1CC1)C | 14 |
100 ,
11

