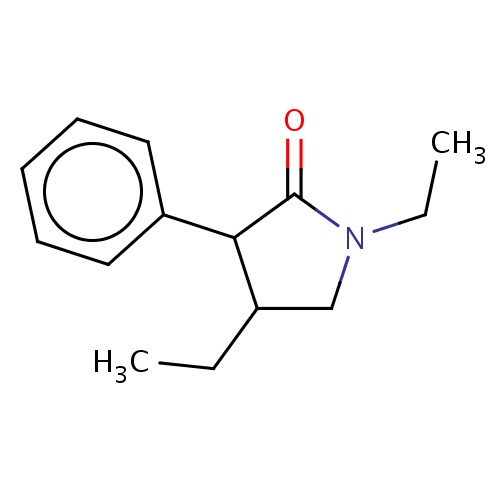
Common name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
IUPAC name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
SMILES
O=C1N(CC(C1c2ccccc2)CC)CC
Common name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
IUPAC name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
SMILES
O=C1N(CC(C1c2ccccc2)CC)CC
INCHI
InChI=1S/C14H19NO/c1-3-11-10-15(4-2)14(16)13(11)12-8-6-5-7-9-12/h5-9,11,13H,3-4,10H2,1-2H3/t11-,13-/m1/s1
FORMULA
C14H19NO

Common name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
IUPAC name
(3R,4S)-1,4-diethyl-3-phenyl-pyrrolidin-2-one
Molecular weight
217.307
clogP
2.812
clogS
-3.105
Frequency
0.0003
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
20.31
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00428 | Doxapram |
|
Central Nervous System Stimulants; Respiratory System Agents; Respiratory System; Respiratory Stimulants; | For use as a temporary measure in hospitalized patients with acute respiratory insufficiency superimposed on chronic obstructive pulmonary disease. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2fv5_ligand_2_13.mol2 | 2fv5 | 0.887097 | -7.42 | c1ccc(cc1)[C@@]1(CCN(CC)C1=O)C | 15 |
| 2fv5_ligand_1_3.mol2 | 2fv5 | 0.854839 | -7.14 | c1ccc(cc1)[C@@]1(CCNC1=O)C | 13 |
| 1mzc_ligand_1_0.mol2 | 1mzc | 0.835821 | -7.09 | c1(ccccc1)[C@H]1C(=O)N(CCCC1)C | 15 |
| 2fv5_ligand_3_17.mol2 | 2fv5 | 0.797101 | -7.47 | Oc1ccc(cc1)[C@@]1(CCN(CC)C1=O)C | 16 |
| 4tw7_ligand_2_122.mol2 | 4tw7 | 0.777778 | -7.35 | c1(ccccc1)CC(=O)N1CCCCC1 | 15 |
| 4tw8_ligand_2_139.mol2 | 4tw8 | 0.777778 | -7.19 | C(C(=O)N1CCCCC1)c1ccccc1 | 15 |
| 1eb2_ligand_2_6.mol2 | 1eb2 | 0.777778 | -6.02 | c1c(cccc1)CC(=O)N1CCCCC1 | 15 |
| 2fv5_ligand_2_9.mol2 | 2fv5 | 0.768116 | -7.19 | Oc1ccc(cc1)[C@@]1(CCNC1=O)C | 14 |
| 4zxx_ligand_2_8.mol2 | 4zxx | 0.758065 | -6.27 | C(c1ccccc1)C(=O)N1CCCC1 | 14 |
| 4x8v_ligand_2_1.mol2 | 4x8v | 0.758065 | -6.14 | c1ccccc1CC(=O)N1CCCC1 | 14 |
109 ,
11

