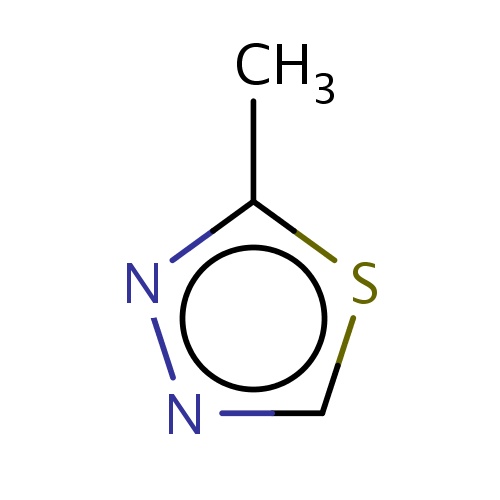
Common name
2-methyl-1,3,4-thiadiazole
IUPAC name
2-methyl-1,3,4-thiadiazole
SMILES
s1cnnc1C
Common name
2-methyl-1,3,4-thiadiazole
IUPAC name
2-methyl-1,3,4-thiadiazole
SMILES
s1cnnc1C
INCHI
InChI=1S/C3H4N2S/c1-3-5-4-2-6-3/h2H,1H3
FORMULA
C3H4N2S

Common name
2-methyl-1,3,4-thiadiazole
IUPAC name
2-methyl-1,3,4-thiadiazole
Molecular weight
100.142
clogP
2.347
clogS
-1.078
Frequency
0.0007
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
54.02
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
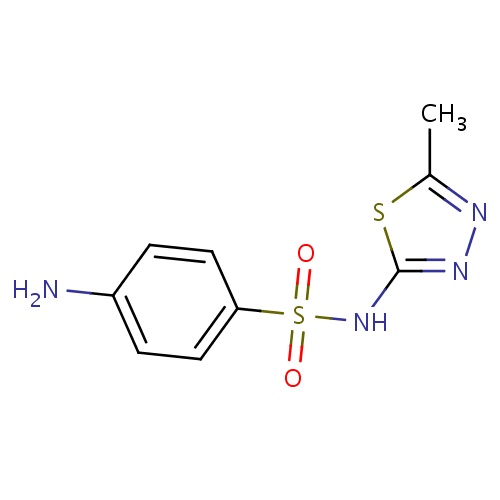
| FDBD00442 | Sulfamethizole |
|
Anti-Infective Agents; Sulfonamides; Blood and Blood Forming Organs; Ophthalmologicals; Sensory Organs; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Dermatologicals; Blood Substitutes and Perfusion Solutions; Irrigating Solutions; Antiinfectives; Short-Acting Sulfonamides; Sulfonamides and Trimethoprim; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; | For the treatment of urinary tract infection. |
| FDBD01133 | Cefazolin |

|
Anti-Bacterial Agents; Cephalosporins; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Antibacterials for Intramammary Use; First-Generation Cephalosporins; | Mainly used to treat bacterial infections of the skin. It can also be used to treat moderately severe bacterial infections involving the lung, bone, joint, stomach, blood, heart valve, and urinary tract. It is clinically effective against infections caused by staphylococci and streptococci species of Gram positive bacteria. May be used for surgical prophylaxis; if required metronidazole may be added to cover B. fragilis. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5dd9_ligand_frag_4.mol2 | 5dd9 | 1 | -5.58 | c1sc(nn1)C | 6 |
| 3bl0_ligand_1_1.mol2 | 3bl0 | 1 | -5.55 | Cc1scnn1 | 6 |
| 5dda_ligand_1_4.mol2 | 5dda | 0.69697 | -5.35 | c1sc(nn1)CF | 7 |
| 5ddb_ligand_1_4.mol2 | 5ddb | 0.676471 | -5.46 | C(F)(F)c1scnn1 | 8 |
| 3dcc_ligand_frag_1.mol2 | 3dcc | 0.608696 | -5.42 | c1scnn1 | 5 |
123 ,
13

