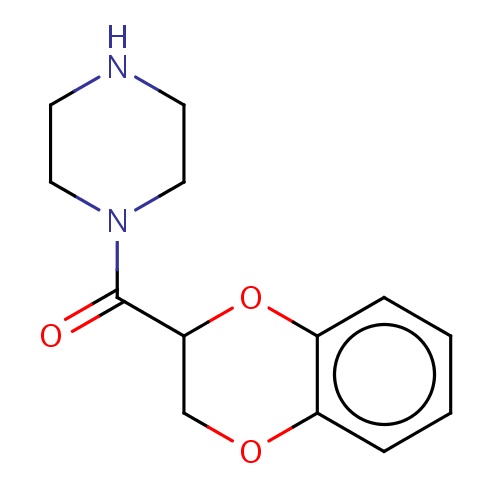
Common name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
IUPAC name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
SMILES
O1C(COc2c1cccc2)C(=O)N3CCNCC3
Common name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
IUPAC name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
SMILES
O1C(COc2c1cccc2)C(=O)N3CCNCC3
INCHI
InChI=1S/C13H16N2O3/c16-13(15-7-5-14-6-8-15)12-9-17-10-3-1-2-4-11(10)18-12/h1-4,12,14H,5-9H2/t12-/m1/s1
FORMULA
C13H16N2O3

Common name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
IUPAC name
[(3R)-2,3-dihydro-1,4-benzodioxin-3-yl]-piperazin-1-yl-methanone
Molecular weight
248.278
clogP
1.198
clogS
-2.045
Frequency
0.0003
HBond Acceptor
3
HBond Donor
1
Total PolarSurface Area
50.8
Number of Rings
3
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00455 | Doxazosin |
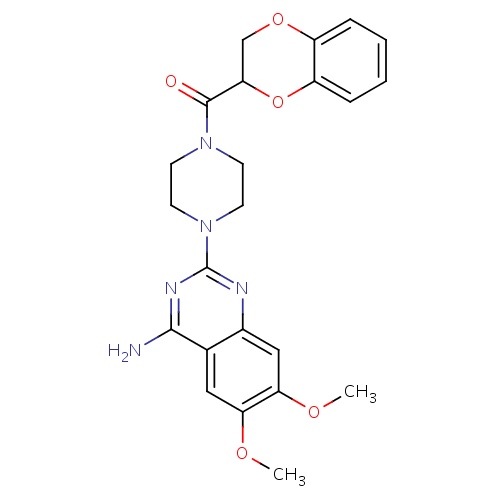
|
Antihypertensive Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Cardiovascular System; Antiadrenergic Agents, Peripherally Acting; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For treatment and management of mild to moderate hypertension and urinary obstruction symptoms caused by BPH. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4yv0_ligand_1_1.mol2 | 4yv0 | 0.847826 | -7.48 | C(=O)(NC)[C@@H]1COc2c(cccc2)O1 | 14 |
| 5alg_ligand_2_8.mol2 | 5alg | 0.71875 | -7.04 | C([C@H]1COc2c(cccc2)O1)NC=O | 14 |
| 4yv0_ligand.mol2 | 4yv0 | 0.702703 | -9.07 | c1ccc(cc1)N(C(=O)[C@@H]1COc2ccccc2O1)C | 21 |
| 4de5_ligand.mol2 | 4de5 | 0.614583 | -7.13 | O=C(O)[C@H]1Oc2ccccc2OC1 | 14 |
| 4g5f_ligand.mol2 | 4g5f | 0.614583 | -7.12 | O=C(O)[C@H]1Oc2ccccc2OC1 | 14 |
| 1tft_ligand_3_39.mol2 | 1tft | 0.524752 | -6.16 | O(c1ccccc1)[C@@H]1CN(CC1)C(=O)C | 15 |
| 5alg_ligand_1_2.mol2 | 5alg | 0.51087 | -7.00 | C[C@H]1COc2c(cccc2)O1 | 11 |
| 1tft_ligand_2_21.mol2 | 1tft | 0.5 | -5.97 | O(c1ccccc1)[C@@H]1CN(CC1)C=O | 14 |
| 5alm_ligand_3_5.mol2 | 5alm | 0.489583 | -7.55 | C[N@@H+]1CC[C@@](C1)(C)Oc1ccccc1 | 14 |
| 5alm_ligand_2_2.mol2 | 5alm | 0.489583 | -7.53 | C1C[NH2+]C[C@]1(C)Oc1ccccc1 | 13 |
100 ,
11

