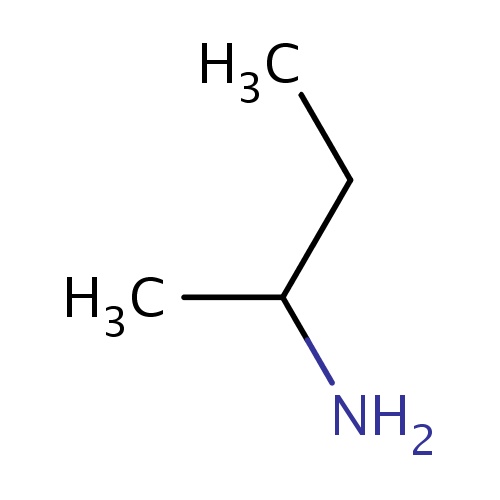
Common name
(2S)-butan-2-amine
IUPAC name
(2S)-butan-2-amine
SMILES
CCC(N)C
Common name
(2S)-butan-2-amine
IUPAC name
(2S)-butan-2-amine
SMILES
CCC(N)C
INCHI
InChI=1S/C4H11N/c1-3-4(2)5/h4H,3,5H2,1-2H3/t4-/m0/s1
FORMULA
C4H11N

Common name
(2S)-butan-2-amine
IUPAC name
(2S)-butan-2-amine
Molecular weight
73.137
clogP
0.027
clogS
-0.612
Frequency
0.0010
HBond Acceptor
0
HBond Donor
2
Total PolarSurface Area
26.02
Number of Rings
0
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00463 | Labetalol |
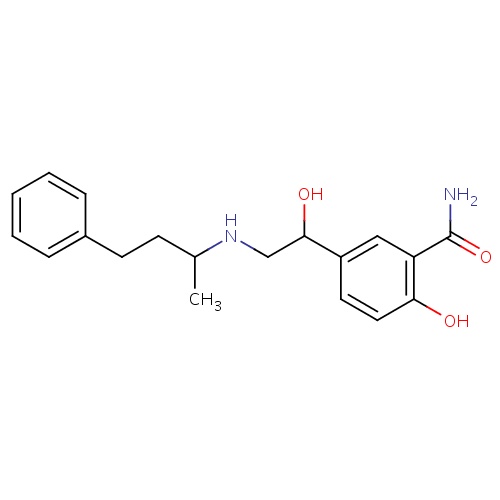
|
Antihypertensive Agents; Sympathomimetics; Adrenergic beta-Antagonists; Cardiovascular System; Beta Blocking Agents; Beta Blocking Agents and Thiazides; Alpha and Beta Blocking Agents; Alpha and Beta Blocking Agents and Thiazides; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the management of hypertension (alone or in combination with other classes of antihypertensive agents), as well as chronic stable angina pectoris and sympathetic overactivity syndrome associated with severe tetanus. Labetalol is used parenterally for immediate reduction in blood pressure in severe hypertension or in hypertensive crises when considered an emergency, for the control of blood pressure in patients with pheochromocytoma and pregnant women with preeclampsia, and to produce controlled hypotension during anesthesia to reduce bleeding resulting from surgical procedures. |
| FDBD00473 | Chloroquine |
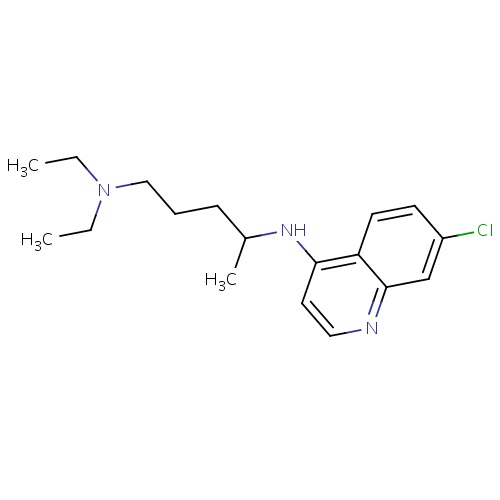
|
Antirheumatic Agents; Antimalarials; Antiprotozoal Agents; Amebicides; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the suppressive treatment and for acute attacks of malaria due to P. vivax, P.malariae, P. ovale, and susceptible strains of P. falciparum, Second-line agent in treatment of Rheumatoid Arthritis. |
| FDBD00953 | Quinacrine |
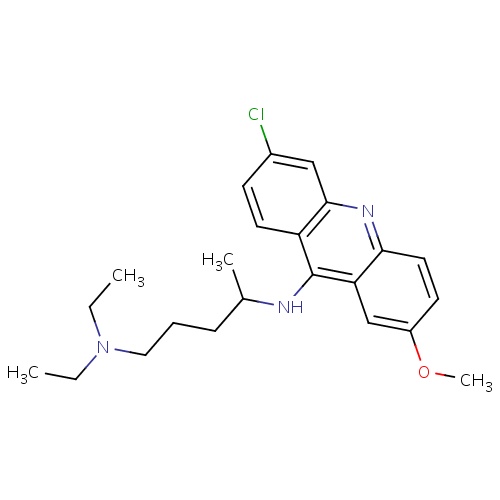
|
Antineoplastic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antinematodal Agents; Anthelmintics; Anticestodal Agents; Antiparasitic Products, Insecticides and Repellents; Agents Against Protozoal Diseases; CYP3A4 Inhibitors; | For the treatment of giardiasis and cutaneous leishmaniasis and the management of malignant effusions. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4fmu_ligand_3_21.mol2 | 4fmu | 1 | -6.26 | [C@@H]([NH3+])(C)CC | 5 |
| 2fdp_ligand_3_78.mol2 | 2fdp | 1 | -6.18 | C([C@H](C)[NH3+])C | 5 |
| 2oah_ligand_3_164.mol2 | 2oah | 1 | -6.17 | C([C@H](C)[NH3+])C | 5 |
| 1qaq_ligand_3_10.mol2 | 1qaq | 1 | -6.11 | C[C@H](CC)[NH3+] | 5 |
| 2oah_ligand_3_171.mol2 | 2oah | 1 | -6.10 | C[C@H](CC)[NH3+] | 5 |
| 3cbp_ligand_3_10.mol2 | 3cbp | 1 | -6.04 | C[C@H](CC)[NH3+] | 5 |
| 3bx5_ligand_2_9.mol2 | 3bx5 | 1 | -5.98 | C(C)[C@@H](C)[NH3+] | 5 |
| 2h2j_ligand_3_10.mol2 | 2h2j | 1 | -5.94 | C[C@H](CC)[NH3+] | 5 |
| 1jqe_ligand_3_49.mol2 | 1jqe | 1 | -5.90 | CC[C@@H](C)[NH3+] | 5 |
| 4awo_ligand_2_0.mol2 | 4awo | 1 | -5.86 | C(C)[C@H]([NH3+])C | 5 |
331 ,
34

