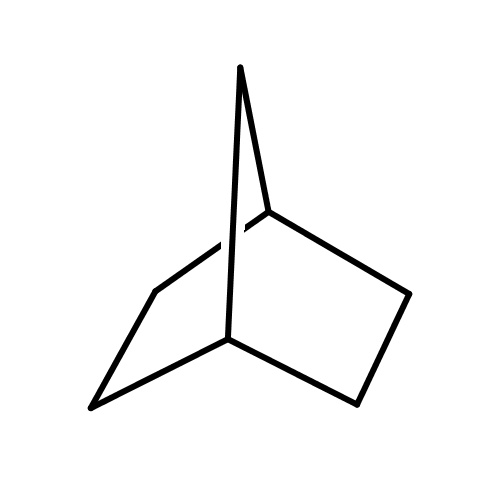
Common name
norbornane
IUPAC name
norbornane
SMILES
C1C2CC(C1)CC2
Common name
norbornane
IUPAC name
norbornane
SMILES
C1C2CC(C1)CC2
INCHI
InChI=1S/C7H12/c1-2-7-4-3-6(1)5-7/h6-7H,1-5H2/t6-,7+
FORMULA
C7H12

Common name
norbornane
IUPAC name
norbornane
Molecular weight
96.170
clogP
2.455
clogS
-1.271
Frequency
0.0010
HBond Acceptor
0
HBond Donor
0
Total PolarSurface Area
0
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00471 | Cyclothiazide |

|
Antihypertensive Agents; Diuretics; Cardiovascular System; Thiazides, Plain; Low-Ceiling Diuretics, Thiazides; | Cyclothiazide is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. It is also indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension. |
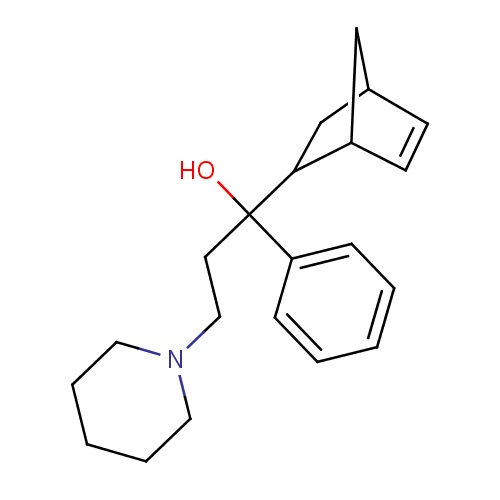
| FDBD00672 | Biperiden |
|
Antiparkinson Agents; Muscarinic Antagonists; Parasympatholytics; Antidyskinetics; Nervous System; Anti-Parkinson Drugs; Anticholinergics; Tertiary Amines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For use as an adjunct in the therapy of all forms of parkinsonism and control of extrapyramidal disorders secondary to neuroleptic drug therapy. |
| FDBD01214 | Fencamfamine |

|
Central Nervous System Stimulants; Antipsychotic Agents; Nervous System; Psychoanaleptics; Centrally Acting Sympathomimetics; Psychostimulants, Agents Used for Adhd and Nootropics; | For the the treatment of depressive fatigue in convalescence and other debilitated states as well as in the treatment of depressive day-time fatigue, lack of concentration and lethargy. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4fk6_ligand_frag_1.mol2 | 4fk6 | 1 | -6.37 | C1C[C@H]2C[C@@H]1CC2 | 7 |
| 4xnw_ligand_1_2.mol2 | 4xnw | 0.888889 | -6.13 | C[C@]12CCC[C@H]1C2 | 7 |
| 2zmj_ligand_frag_1.mol2 | 2zmj | 0.875 | -7.69 | C1[C@H]2C[C@H]3C[C@@H]1C[C@H](C3)C2 | 10 |
| 5alz_ligand_frag_2.mol2 | 5alz | 0.875 | -7.67 | [C@@H]12C[C@H]3C[C@@H](C1)C[C@H](C3)C2 | 10 |
| 3hy5_ligand_frag_0.mol2 | 3hy5 | 0.875 | -7.64 | C1(CCC[C@H](C1)C)(C)C | 9 |
| 4ciz_ligand_frag_0.mol2 | 4ciz | 0.875 | -7.58 | C1(CCC[C@@H](C1)C)(C)C | 9 |
| 4y2x_ligand_frag_1.mol2 | 4y2x | 0.875 | -7.55 | C1[C@H]2C[C@H]3C[C@@H]1C[C@@H](C2)C3 | 10 |
| 5am3_ligand_frag_3.mol2 | 5am3 | 0.875 | -7.48 | C1[C@@H]2C[C@@H]3C[C@H]1C[C@H](C2)C3 | 10 |
265 ,
27

