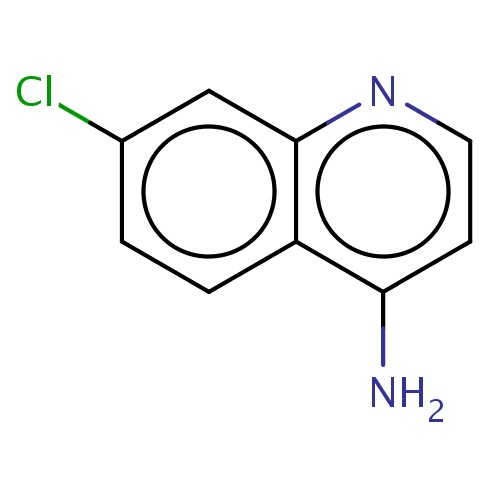
Common name
7-chloroquinolin-4-amine
IUPAC name
7-chloroquinolin-4-amine
SMILES
Clc1cc2nccc(c2cc1)N
Common name
7-chloroquinolin-4-amine
IUPAC name
7-chloroquinolin-4-amine
SMILES
Clc1cc2nccc(c2cc1)N
INCHI
InChI=1S/C9H7ClN2/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H,(H2,11,12)
FORMULA
C9H7ClN2

Common name
7-chloroquinolin-4-amine
IUPAC name
7-chloroquinolin-4-amine
Molecular weight
178.618
clogP
2.361
clogS
-3.199
Frequency
0.0010
HBond Acceptor
1
HBond Donor
2
Total Polar
Surface Area
38.91
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
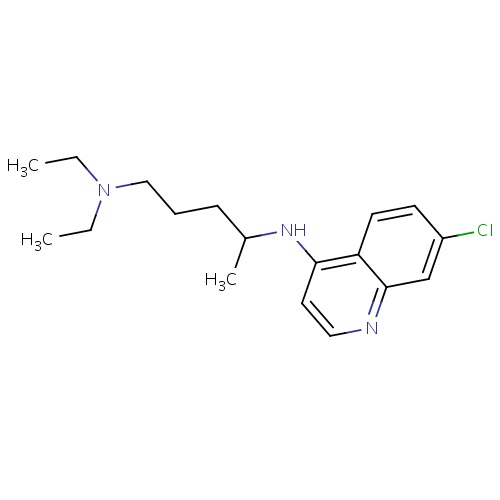
| FDBD00473 | Chloroquine |
|
Antirheumatic Agents; Antimalarials; Antiprotozoal Agents; Amebicides; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the suppressive treatment and for acute attacks of malaria due to P. vivax, P.malariae, P. ovale, and susceptible strains of P. falciparum, Second-line agent in treatment of Rheumatoid Arthritis. |
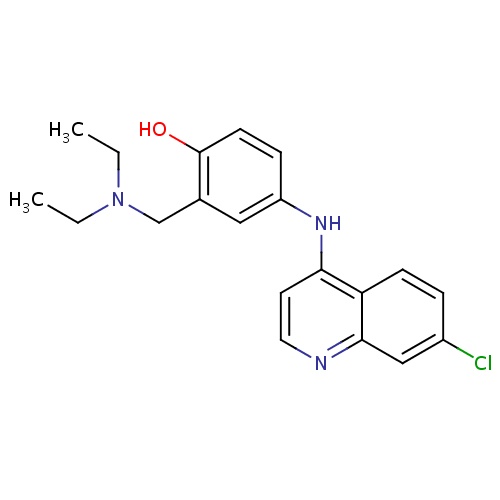
| FDBD00478 | Amodiaquine |
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For treatment of acute malarial attacks in non-immune subjects. |
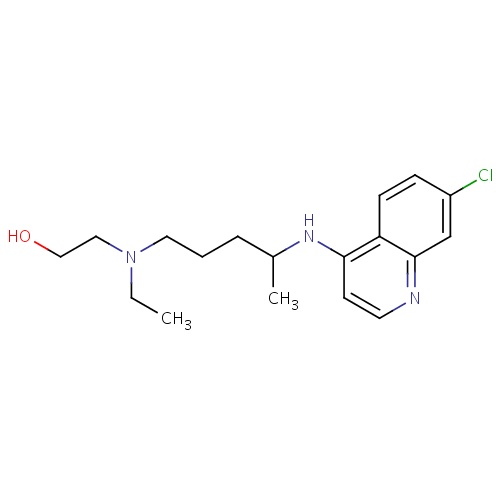
| FDBD01261 | Hydroxychloroquine |
|
Antirheumatic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the suppressive treatment and treatment of acute attacks of malaria due to . |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1cet_ligand_1_0.mol2 | 1cet | 1 | -7.17 | Nc1c2c(ncc1)cc(cc2)Cl | 12 |
| 2aou_ligand_1_0.mol2 | 2aou | 1 | -7.05 | Nc1c2ccc(cc2ncc1)Cl | 12 |
| 4fgz_ligand_1_0.mol2 | 4fgz | 1 | -5.85 | Nc1c2ccc(cc2ncc1)Cl | 12 |
| 2ym8_ligand_1_3.mol2 | 2ym8 | 0.782609 | -7.29 | Clc1cccc2cc(ncc12)N | 12 |
| 3fue_ligand.mol2 | 3fue | 0.714286 | -7.26 | c1cc2cc(ccc2[nH]1)Cl | 11 |
| 2xui_ligand_1_11.mol2 | 2xui | 0.692308 | -8.04 | c1(c2ccccc2nc2ccccc12)N | 15 |
| 2xup_ligand_1_11.mol2 | 2xup | 0.692308 | -8.03 | Nc1c2ccccc2nc2ccccc12 | 15 |
| 4bds_ligand.mol2 | 4bds | 0.692308 | -7.91 | c1cccc2nc3ccccc3c(c12)N | 16 |
185 ,
19

