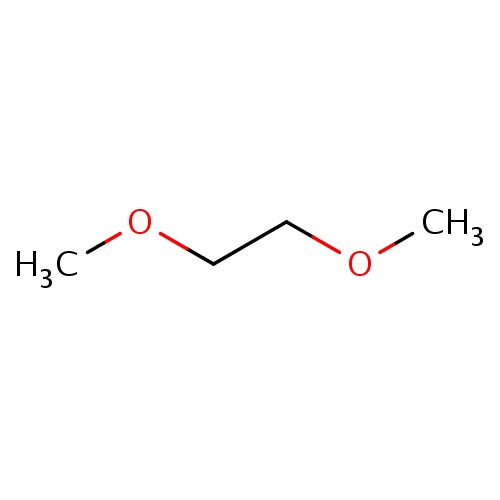
Common name
1,2-dimethoxyethane
IUPAC name
1,2-dimethoxyethane
SMILES
C(OC)COC
Common name
1,2-dimethoxyethane
IUPAC name
1,2-dimethoxyethane
SMILES
C(OC)COC
INCHI
InChI=1S/C4H10O2/c1-5-3-4-6-2/h3-4H2,1-2H3
FORMULA
C4H10O2

Common name
1,2-dimethoxyethane
IUPAC name
1,2-dimethoxyethane
Molecular weight
90.121
clogP
0.277
clogS
-0.847
Frequency
0.0027
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
18.46
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00481 | Candoxatril |
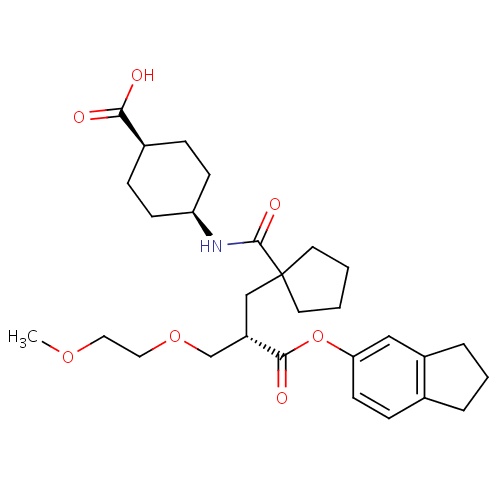
|
Prodrugs; | For treatment of hypertension, improve exercise capacity in patients with CHF receiving angiotensin converting enzyme inhibition. |
| FDBD00641 | Roxithromycin |

|
Anti-Bacterial Agents; Macrolides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | Used to treat respiratory tract, urinary and soft tissue infections. |
| FDBD00809 | Dirithromycin |

|
Anti-Infective Agents; Macrolides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; | For the treatment of the following mild-to-moderate infections caused by susceptible strains of microorganisms: acute bacterial exacerbations of chronic bronchitis, secondary bacterial infection of acute bronchitis, community-acquired pneumonia, pharyngitis/tonsilitis, and uncomplicated skin and skin structure infections. |
| FDBD01665 | Vilanterol |
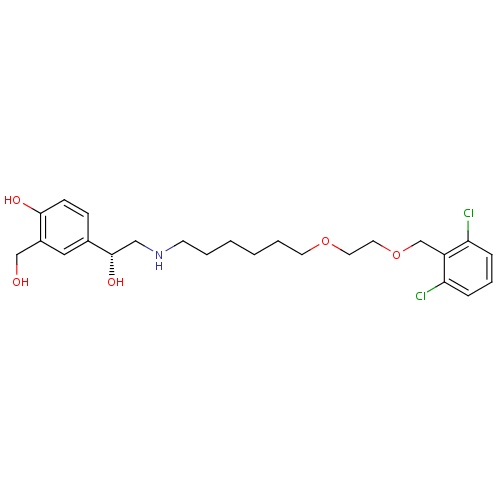
|
Immunosuppressive Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Adrenergics, Inhalants; CYP3A4 Inhibitors; Beta2 Agonists; | Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta and in combination with umeclidinium bromide as Anoro Ellipta. Approved by the FDA in 2013, use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema. It is also indicated for once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. |
| FDBD01715 | Florbetaben (18F) |
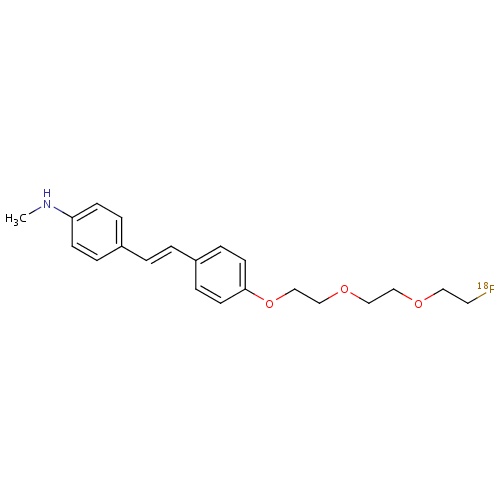
|
Diagnostic Radiopharmaceuticals; Central Nervous System; | |
| FDBD01716 | Florbetapir (18F) |
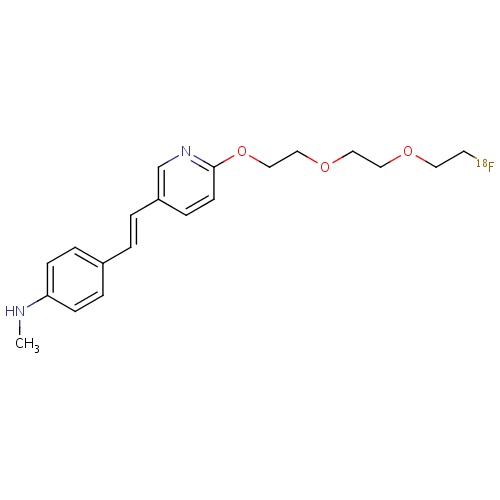
|
Diagnostic Radiopharmaceuticals; Central Nervous System; | |
| FDBD01822 | Piperonyl butoxide |
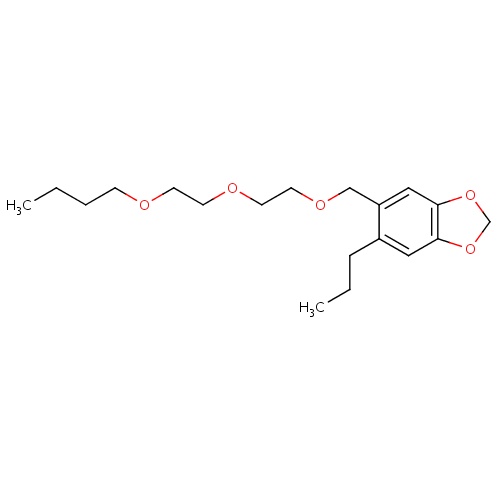
|
Pesticide Synergists; | For the treatment of head, pubic (crab), and body lice. |
| FDBD02849 | bicyclopyrone |
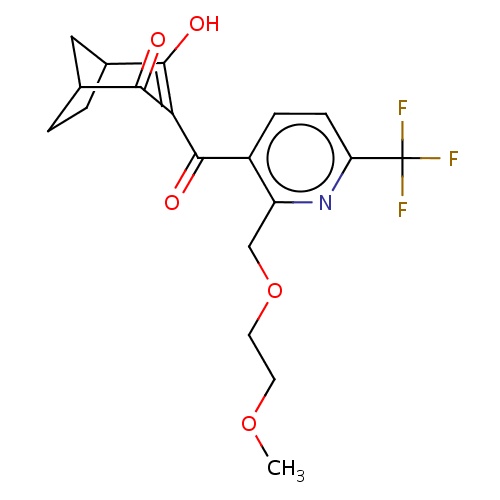
|
Herbicide | Herbicide |
8 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1ikt_ligand_5_1266.mol2 | 1ikt | 1 | -5.42 | C(OC)COC | 6 |
| 1ikt_ligand_4_714.mol2 | 1ikt | 1 | -5.18 | O(C)CCOC | 6 |
| 2bvs_ligand_5_4362.mol2 | 2bvs | 1 | -4.94 | O(C)CCOC | 6 |
| 2xei_ligand_5_8512.mol2 | 2xei | 1 | -4.84 | O(C)CCOC | 6 |
| 1cnx_ligand_5_246.mol2 | 1cnx | 1 | -4.80 | C(COC)OC | 6 |
| 1cny_ligand_5_1750.mol2 | 1cny | 1 | -4.79 | C(COC)OC | 6 |
| 1em6_ligand_5_105.mol2 | 1em6 | 1 | -4.75 | COCCOC | 6 |
| 1cnw_ligand_5_736.mol2 | 1cnw | 1 | -4.71 | COCCOC | 6 |
101 ,
11

