
Common name
ethyl 2-hydroxy-2-methyl-propanoate
IUPAC name
ethyl 2-hydroxy-2-methyl-propanoate
SMILES
OC(C)(C)C(=O)OCC
Common name
ethyl 2-hydroxy-2-methyl-propanoate
IUPAC name
ethyl 2-hydroxy-2-methyl-propanoate
SMILES
OC(C)(C)C(=O)OCC
INCHI
InChI=1S/C6H12O3/c1-4-9-5(7)6(2,3)8/h8H,4H2,1-3H3
FORMULA
C6H12O3

Common name
ethyl 2-hydroxy-2-methyl-propanoate
IUPAC name
ethyl 2-hydroxy-2-methyl-propanoate
Molecular weight
132.158
clogP
0.252
clogS
-0.574
Frequency
0.0003
HBond Acceptor
3
HBond Donor
1
Total PolarSurface Area
46.53
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00501 | Clofibrate |
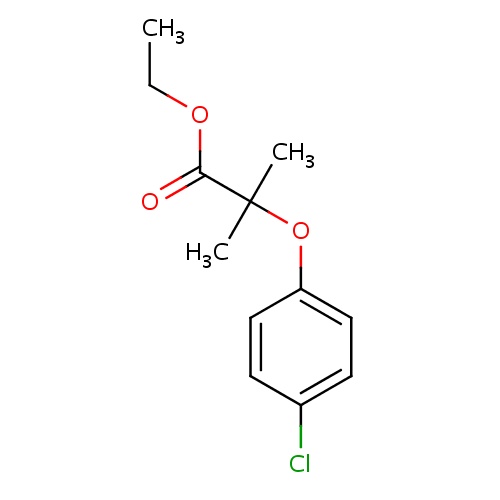
|
Anticholesteremic Agents; Hypolipidemic Agents; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Fibrates; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2B6 Inhibitors (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | For Primary Dysbetalipoproteinemia (Type III hyperlipidemia) that does not respond adequately to diet. This helps control high cholesterol and high triglyceride levels. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1g37_ligand_3_50.mol2 | 1g37 | 1 | -5.51 | C[C@@H](C(=O)OCC)O | 8 |
| 3e3c_ligand_3_36.mol2 | 3e3c | 0.818182 | -6.10 | C[C@@H](O)COC(=O)C | 8 |
| 4xpj_ligand_3_404.mol2 | 4xpj | 0.818182 | -5.35 | [C@H](O)(C)COC(=O)C | 8 |
| 1p6d_ligand_4_1325.mol2 | 1p6d | 0.8 | -6.51 | C(OC(=O)CC)(C)C | 8 |
| 1p6e_ligand_4_671.mol2 | 1p6e | 0.8 | -6.42 | CC(C)OC(=O)CC | 8 |
| 1p6d_ligand_3_395.mol2 | 1p6d | 0.8 | -6.18 | C(OC(=O)CC)C | 7 |
| 1p6e_ligand_3_244.mol2 | 1p6e | 0.8 | -6.12 | CCOC(=O)CC | 7 |
| 1p6d_ligand_3_350.mol2 | 1p6d | 0.8 | -6.09 | C(OC(=O)CC)C | 7 |
| 4tnw_ligand_3_158.mol2 | 4tnw | 0.8 | -5.99 | CCC(=O)OCC | 7 |
| 1p6e_ligand_3_208.mol2 | 1p6e | 0.8 | -5.97 | CCOC(=O)CC | 7 |
133 ,
14

