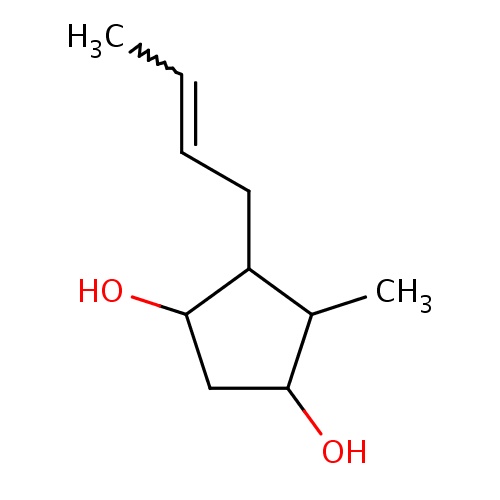
Common name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
IUPAC name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
SMILES
CC=CCC1C(C(CC1O)O)C
Common name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
IUPAC name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
SMILES
CC=CCC1C(C(CC1O)O)C
INCHI
InChI=1S/C10H18O2/c1-3-4-5-8-7(2)9(11)6-10(8)12/h3-4,7-12H,5-6H2,1-2H3/b4-3-/t7-,8-,9-,10+/m1/s1
FORMULA
C10H18O2

Common name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
IUPAC name
(1R,3S,4R,5R)-4-[(Z)-but-2-enyl]-5-methyl-cyclopentane-1,3-diol
Molecular weight
170.249
clogP
1.217
clogS
-0.459
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
40.46
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00519 | Latanoprost |
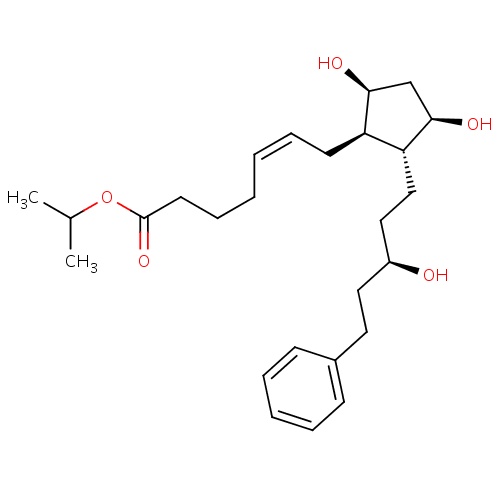
|
Ophthalmologicals; Sensory Organs; Antiglaucoma Preparations and Miotics; Prostaglandin Analogues; | For the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3ihz_ligand_1_4.mol2 | 3ihz | 0.793103 | -6.66 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
| 1fkf_ligand_1_3.mol2 | 1fkf | 0.793103 | -6.43 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
| 1a7x_ligand_1_5.mol2 | 1a7x | 0.793103 | -5.97 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
| 4nnr_ligand_1_4.mol2 | 4nnr | 0.793103 | -5.86 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
| 1qpl_ligand_2_23.mol2 | 1qpl | 0.793103 | -5.80 | [C@@H]1(CC[C@@H](CC1)O)/C=C/C | 10 |
| 1o9e_ligand_2_1.mol2 | 1o9e | 0.764706 | -8.00 | C[C@@H]1C2=C[C@@]3([C@@H]([C@@H](C[C@@H]3O)CC)C[C@@H]([C@@H]([C@@H]2CC1)C)O)C | 21 |
| 1o9e_ligand_1_2.mol2 | 1o9e | 0.764706 | -7.76 | C[C@@H]1C2=C[C@@]3([C@@H](CC[C@@H]3O)C[C@@H]([C@@H]([C@@H]2CC1)C)O)C | 19 |
| 1o9e_ligand_1_0.mol2 | 1o9e | 0.764706 | -7.69 | [C@@H]12[C@@H](C[C@@H]([C@@]1(C=C1CCC[C@H]1[C@H]([C@H](C2)O)C)C)O)CC | 20 |
| 1o9e_ligand_frag_0.mol2 | 1o9e | 0.764706 | -7.45 | [C@@H]12CC[C@@H]([C@@]1(C=C1CCC[C@H]1[C@H]([C@H](C2)O)C)C)O | 18 |
102 ,
11

