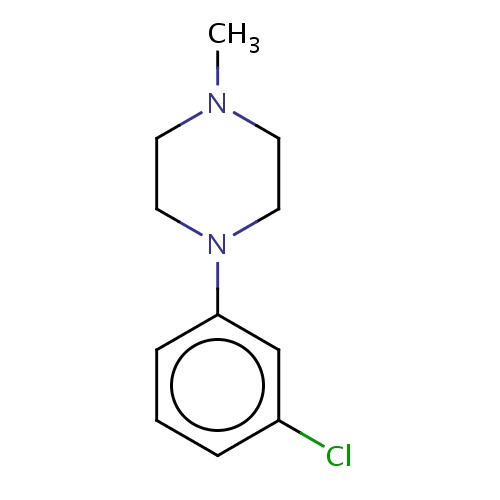
Common name
1-(3-chlorophenyl)-4-methyl-piperazine
IUPAC name
1-(3-chlorophenyl)-4-methyl-piperazine
SMILES
CN1CCN(CC1)c2cc(ccc2)Cl
Common name
1-(3-chlorophenyl)-4-methyl-piperazine
IUPAC name
1-(3-chlorophenyl)-4-methyl-piperazine
SMILES
CN1CCN(CC1)c2cc(ccc2)Cl
INCHI
InChI=1S/C11H15ClN2/c1-13-5-7-14(8-6-13)11-4-2-3-10(12)9-11/h2-4,9H,5-8H2,1H3
FORMULA
C11H15ClN2

Common name
1-(3-chlorophenyl)-4-methyl-piperazine
IUPAC name
1-(3-chlorophenyl)-4-methyl-piperazine
Molecular weight
210.703
clogP
2.156
clogS
-2.583
Frequency
0.0014
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
6.48
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00521 | Trazodone |
|
Antidepressive Agents, Second-Generation; Anti-Anxiety Agents; Serotonin Uptake Inhibitors; Adrenergic alpha-1 Receptor Antagonists; Nervous System; Antidepressants; Psychoanaleptics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of depression. |
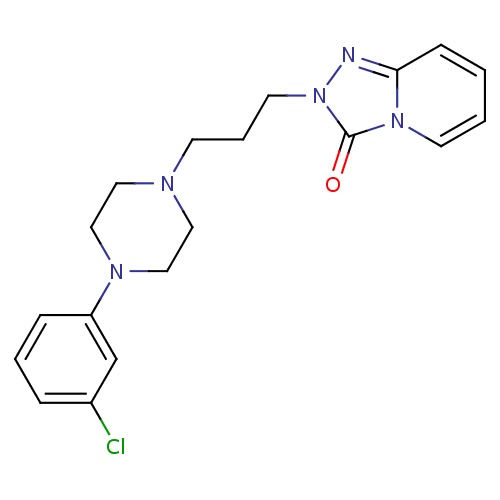
| FDBD00997 | Nefazodone |
|
Antidepressive Agents, Second-Generation; Adrenergic alpha-1 Receptor Antagonists; Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of depression. |
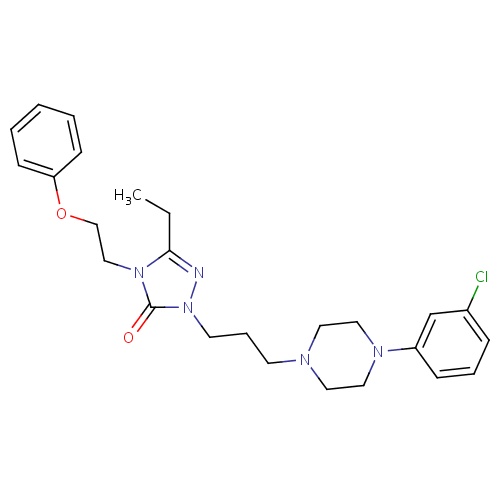
| FDBD01733 | Etoperidone |
|
Nervous System; Antidepressants; Psychoanaleptics; Selective Serotonin Reuptake Inhibitors; | |
| FDBD01735 | Mepiprazole |

|
; | For the treatment of anxiety neuroses. |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3fv8_ligand_2_3.mol2 | 3fv8 | 0.829787 | -7.11 | Clc1ccccc1N1CC[NH+](C)CC1 | 14 |
| 3fv8_ligand_1_1.mol2 | 3fv8 | 0.829787 | -6.98 | Clc1ccccc1N1CC[NH2+]CC1 | 13 |
| 1s64_ligand_2_1.mol2 | 1s64 | 0.781818 | -6.72 | c1(cc(ccc1)Cl)N1CC[N@@H+](CC1=O)C | 15 |
| 1s64_ligand_1_2.mol2 | 1s64 | 0.781818 | -6.68 | c1(cc(ccc1)Cl)N1CC[NH2+]CC1=O | 14 |
| 1s63_ligand_2_1.mol2 | 1s63 | 0.781818 | -6.55 | C[N@H+]1CCN(C(=O)C1)c1cc(ccc1)Cl | 15 |
| 1s63_ligand_1_2.mol2 | 1s63 | 0.781818 | -6.51 | [NH2+]1CCN(C(=O)C1)c1cc(ccc1)Cl | 14 |
| 4ql1_ligand_1_3.mol2 | 4ql1 | 0.744186 | -7.19 | c1ccccc1N1CC[NH+](CC1)C | 13 |
| 4hyi_ligand_1_1.mol2 | 4hyi | 0.744186 | -7.01 | c1cccc(c1)N1CC[NH2+]CC1 | 12 |
108 ,
11

