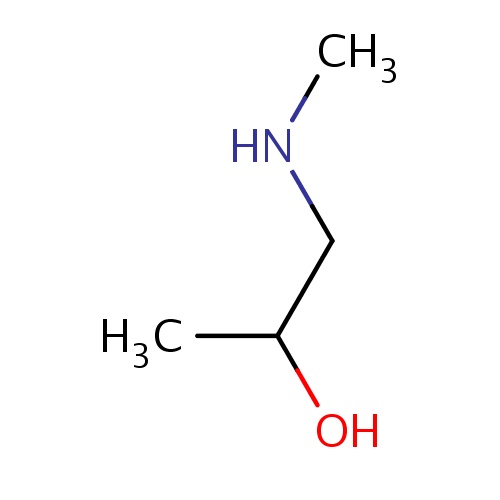
Common name
(2S)-1-(methylamino)propan-2-ol
IUPAC name
(2S)-1-(methylamino)propan-2-ol
SMILES
CNCC(O)C
Common name
(2S)-1-(methylamino)propan-2-ol
IUPAC name
(2S)-1-(methylamino)propan-2-ol
SMILES
CNCC(O)C
INCHI
InChI=1S/C4H11NO/c1-4(6)3-5-2/h4-6H,3H2,1-2H3/t4-/m0/s1
FORMULA
C4H11NO

Common name
(2S)-1-(methylamino)propan-2-ol
IUPAC name
(2S)-1-(methylamino)propan-2-ol
Molecular weight
89.136
clogP
-0.362
clogS
-0.485
Frequency
0.0017
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
32.26
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
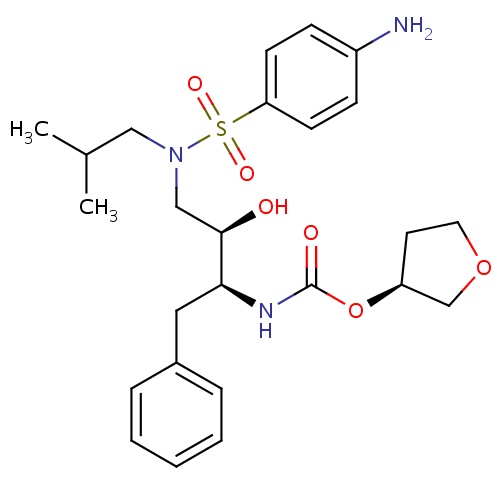
| FDBD00566 | Amprenavir |
|
Anti-HIV Agents; Antibiotics, Antitubercular; Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of HIV-1 infection in combination with other antiretroviral agents. |
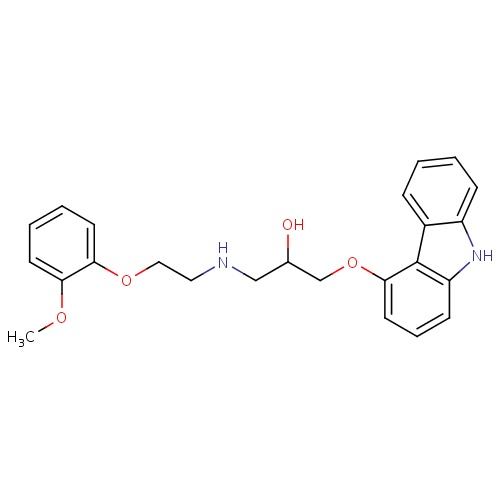
| FDBD00984 | Carvedilol |
|
Antihypertensive Agents; Vasodilator Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic beta-Antagonists; Cardiovascular System; Beta Blocking Agents; Alpha and Beta Blocking Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of mild or moderate (NYHA class II or III) heart failure of ischemic or cardiomyopathic origin. |
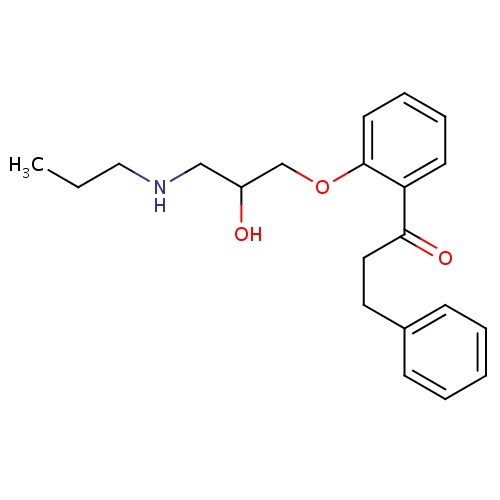
| FDBD01028 | Propafenone |
|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ic; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Used to prolong the time to recurrence of paroxysmal atrial fibrillation/flutter (PAF) associated with disabling symptoms in patients without structural heart disease. Also used for the treatment of life-threatening documented ventricular arrhythmias, such as sustained ventricular tachycardia. |
| FDBD01106 | Darunavir |
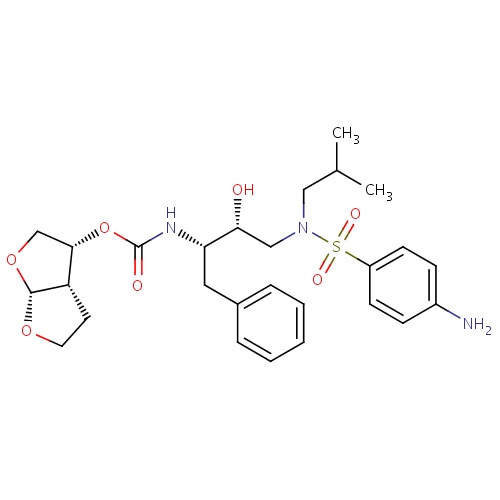
|
Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Darunavir, co-administered with ritonavir, and with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV) infection in antiretroviral treatment-experienced adult patients, such as those with HIV-1 strains resistant to more than one protease inhibitor. |
| FDBD01120 | Bevantolol |
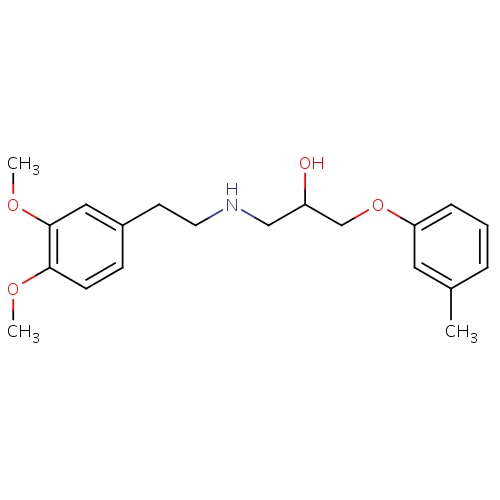
|
Adrenergic beta-1 Receptor Antagonists; Adrenergic alpha-1 Receptor Antagonists; Cardiovascular System; Beta Blocking Agents, Selective; Beta Blocking Agents; Beta Blocking Agents, Selective, and Thiazides; Beta Blocking Agents and Thiazides; | For the treatment of angina pectoris and hypertension. |
5 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vnm_ligand_3_204.mol2 | 2vnm | 1 | -5.95 | C[NH2+]C[C@@H](O)C | 6 |
| 2vnn_ligand_3_104.mol2 | 2vnn | 1 | -5.95 | [C@@H](O)(C[NH2+]C)C | 6 |
| 2wf0_ligand_3_135.mol2 | 2wf0 | 1 | -5.95 | C[C@@H](C[NH2+]C)O | 6 |
| 2vj7_ligand_3_190.mol2 | 2vj7 | 1 | -5.94 | [C@H](O)(C)C[NH2+]C | 6 |
| 2wez_ligand_3_135.mol2 | 2wez | 1 | -5.93 | C[C@H](O)C[NH2+]C | 6 |
| 2wf2_ligand_3_71.mol2 | 2wf2 | 1 | -5.92 | C([NH2+]C)[C@H](C)O | 6 |
| 2wf1_ligand_3_107.mol2 | 2wf1 | 1 | -5.91 | C[C@@H](C[NH2+]C)O | 6 |
| 2ewy_ligand_3_19.mol2 | 2ewy | 1 | -5.90 | C[NH2+]C[C@@H](O)C | 6 |
264 ,
27

