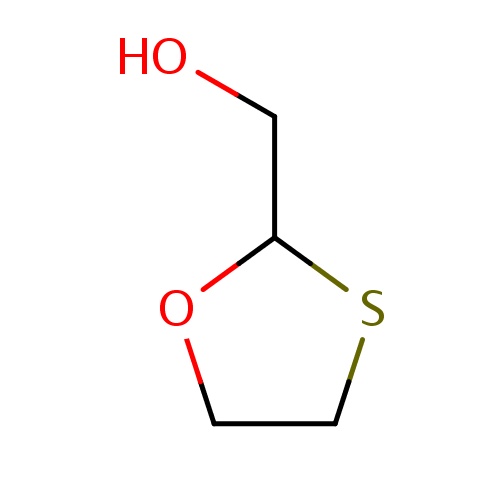
Common name
[(2R)-1,3-oxathiolan-2-yl]methanol
IUPAC name
[(2R)-1,3-oxathiolan-2-yl]methanol
SMILES
S1CCOC1CO
Common name
[(2R)-1,3-oxathiolan-2-yl]methanol
IUPAC name
[(2R)-1,3-oxathiolan-2-yl]methanol
SMILES
S1CCOC1CO
INCHI
InChI=1S/C4H8O2S/c5-3-4-6-1-2-7-4/h4-5H,1-3H2/t4-/m1/s1
FORMULA
C4H8O2S

Common name
[(2R)-1,3-oxathiolan-2-yl]methanol
IUPAC name
[(2R)-1,3-oxathiolan-2-yl]methanol
Molecular weight
120.170
clogP
1.099
clogS
-0.111
Frequency
0.0007
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
54.76
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00573 | Lamivudine |

|
Anti-HIV Agents; Reverse Transcriptase Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Nucleoside and Nucleotide Reverse Transcriptase Inhibitors; | For the treatment of HIV infection and chronic hepatitis B (HBV). |
| FDBD00739 | Emtricitabine |

|
Antiviral Agents; Anti-HIV Agents; Reverse Transcriptase Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Nucleoside and Nucleotide Reverse Transcriptase Inhibitors; | Indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 infection in adults and for postexposure prophylaxis of HIV infection in health care workers and others exposed occupationally or nonoccupationally via percutaneous injury or mucous membrane or nonintact skin contact with blood, tissues, or other body fluids associated with risk for transmission of the virus. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4wkp_ligand_5_231.mol2 | 4wkp | 0.433333 | -5.31 | COCCSC | 6 |
| 4wkp_ligand_5_161.mol2 | 4wkp | 0.413793 | -5.65 | C(C)SCCO | 6 |
| 2fj0_ligand_4_205.mol2 | 2fj0 | 0.413793 | -5.54 | C(CO)SCC | 6 |
104 ,
11

