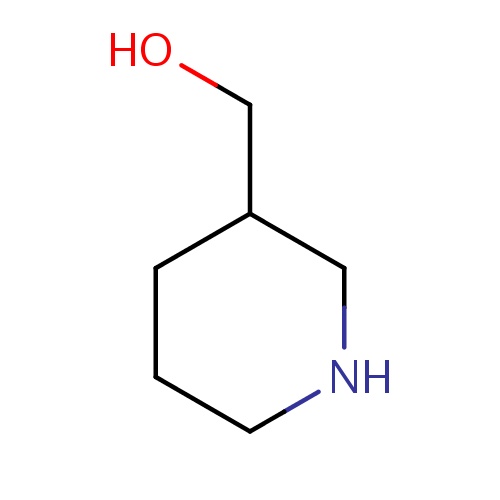
Common name
[(3S)-3-piperidyl]methanol
IUPAC name
[(3S)-3-piperidyl]methanol
SMILES
C(O)C1CNCCC1
Common name
[(3S)-3-piperidyl]methanol
IUPAC name
[(3S)-3-piperidyl]methanol
SMILES
C(O)C1CNCCC1
INCHI
InChI=1S/C6H13NO/c8-5-6-2-1-3-7-4-6/h6-8H,1-5H2/t6-/m0/s1
FORMULA
C6H13NO

Common name
[(3S)-3-piperidyl]methanol
IUPAC name
[(3S)-3-piperidyl]methanol
Molecular weight
115.174
clogP
0.956
clogS
-0.863
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
32.26
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00579 | Paroxetine |

|
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors; Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; Selective Serotonin Reuptake Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); | Labeled indications include: major depressive disorder (MDD), panic disorder with or without agoraphobia, obsessive-compulsive disorder (OCD), social anxiety disorder (social phobia), generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and premenstrual dysphoric disorder (PMDD). Unlabeled indications include: eating disorders, impulse control disorders, vasomotor symptoms of menopause, obsessive-compulsive disorder (OCD) in children, and mild dementia-associated agitation in nonpsychotic individuals. Brisdelle, which consists of paroxetine mesylate is indicated for the treatment of moderate to severe vasomotor symptoms (like hot flashes) associated with menopause. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4mm4_ligand_2_0.mol2 | 4mm4 | 1 | -6.39 | C1CC[C@@H](C[NH2+]1)CO | 8 |
| 4mk0_ligand_2_3.mol2 | 4mk0 | 1 | -5.89 | C1CC[NH2+]C[C@H]1CO | 8 |
| 3d0e_ligand_2_9.mol2 | 3d0e | 1 | -5.85 | OC[C@@H]1C[NH2+]CCC1 | 8 |
| 4l9i_ligand_2_0.mol2 | 4l9i | 1 | -5.53 | OC[C@H]1CCC[NH2+]C1 | 8 |
| 4da5_ligand_frag_0.mol2 | 4da5 | 0.964286 | -6.53 | C(O)[C@H]1C[N@H+]2CC[C@@H]1CC2 | 10 |
| 2cvd_ligand_4_65.mol2 | 2cvd | 0.857143 | -6.04 | [C@@H]1(CC[N@H+](CC1)CCC)O | 10 |
| 1aid_ligand_3_4.mol2 | 1aid | 0.857143 | -5.57 | C([N@H+]1CC[C@H](CC1)O)CC | 10 |
| 4og6_ligand_4_30.mol2 | 4og6 | 0.857143 | -5.57 | C(CO)C[NH+]1CCCCC1 | 10 |
100 ,
11

