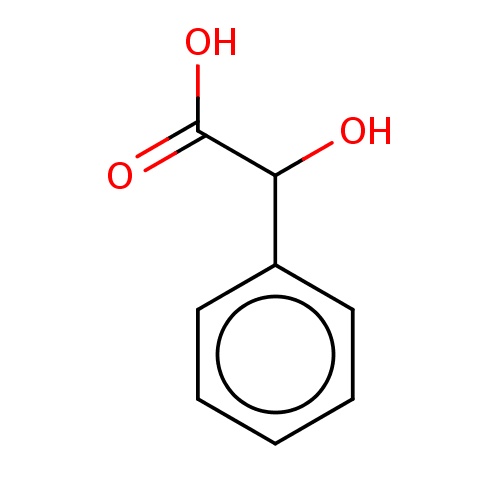
Common name
(2S)-2-hydroxy-2-phenyl-acetic acid
IUPAC name
(2S)-2-hydroxy-2-phenyl-acetic acid
SMILES
OC(=O)C(O)c1ccccc1
Common name
(2S)-2-hydroxy-2-phenyl-acetic acid
IUPAC name
(2S)-2-hydroxy-2-phenyl-acetic acid
SMILES
OC(=O)C(O)c1ccccc1
INCHI
InChI=1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m0/s1
FORMULA
C8H8O3

Common name
(2S)-2-hydroxy-2-phenyl-acetic acid
IUPAC name
(2S)-2-hydroxy-2-phenyl-acetic acid
Molecular weight
152.147
clogP
0.756
clogS
-0.470
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
57.53
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
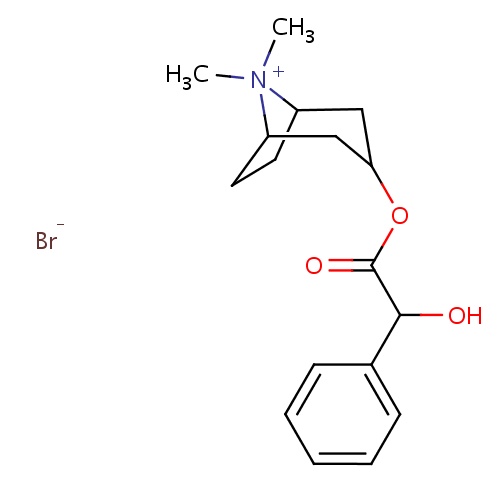
| FDBD00589 | Homatropine Methylbromide |
|
Muscarinic Antagonists; Antispasmodics; Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; | Used in conjunction with antacids or histamine H2-receptor antagonists in the treatment of peptic ulcers, gastric ulcers and duodenal ulcers, to reduce further gastric acid secretion and delay gastric emptying. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1mns_ligand_frag_0.mol2 | 1mns | 1 | -7.55 | c1cc(ccc1)[C@](O)(C(=O)O)C | 12 |
| 1mns_ligand.mol2 | 1mns | 1 | -7.55 | c1cc(ccc1)[C@](O)(C(=O)O)C | 13 |
| 2pj1_ligand_2_33.mol2 | 2pj1 | 1 | -7.16 | c1(ccccc1)[C@@H](C(=O)O)O | 11 |
| 4hnc_ligand_1_0.mol2 | 4hnc | 1 | -7.07 | c1(ccccc1)[C@H](O)C(=O)O | 11 |
| 2pj5_ligand_2_63.mol2 | 2pj5 | 1 | -7.06 | c1(ccccc1)[C@@H](C(=O)O)O | 11 |
| 2pj0_ligand_2_33.mol2 | 2pj0 | 1 | -7.03 | c1(ccccc1)[C@H](O)C(=O)O | 11 |
| 2pj3_ligand_2_42.mol2 | 2pj3 | 1 | -7.02 | O[C@@H](c1ccccc1)C(=O)O | 11 |
| 2pjc_ligand_2_75.mol2 | 2pjc | 1 | -7.01 | c1(ccccc1)[C@@H](C(=O)O)O | 11 |
| 2pj4_ligand_2_33.mol2 | 2pj4 | 1 | -6.96 | O[C@H](C(=O)O)c1ccccc1 | 11 |
| 4hnc_ligand_1_1.mol2 | 4hnc | 1 | -6.78 | [C@H](O)(C(=O)O)c1ccccc1 | 11 |
103 ,
11

