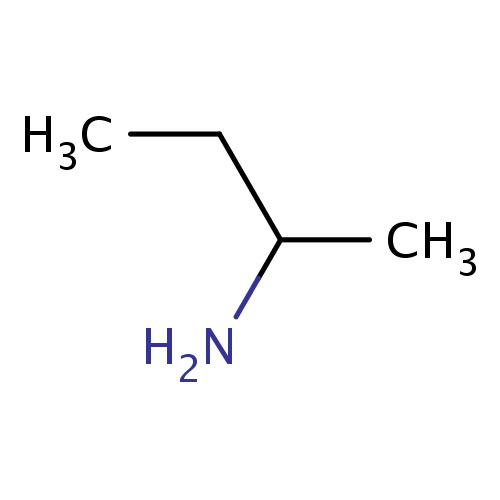
Common name
(2R)-butan-2-amine
IUPAC name
(2R)-butan-2-amine
SMILES
C(C)(N)CC
Common name
(2R)-butan-2-amine
IUPAC name
(2R)-butan-2-amine
SMILES
C(C)(N)CC
INCHI
InChI=1S/C4H11N/c1-3-4(2)5/h4H,3,5H2,1-2H3/t4-/m1/s1
FORMULA
C4H11N

Common name
(2R)-butan-2-amine
IUPAC name
(2R)-butan-2-amine
Molecular weight
73.137
clogP
0.027
clogS
-0.612
Frequency
0.0021
HBond Acceptor
0
HBond Donor
2
Total PolarSurface Area
26.02
Number of Rings
0
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00702 | Dobutamine |
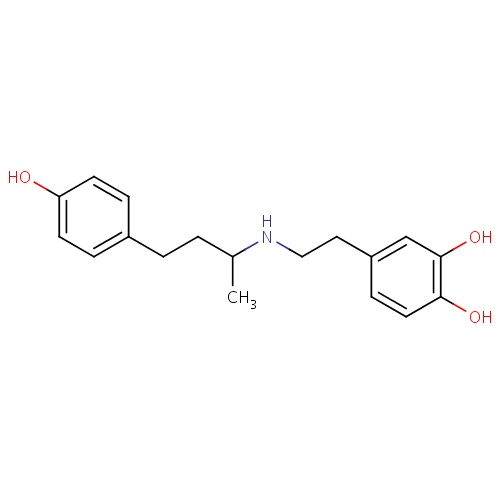
|
Sympathomimetics; Cardiotonic Agents; Adrenergic beta-1 Receptor Agonists; Cardiovascular System; Cardiac Therapy; Adrenergic and Dopaminergic Agents; Cardiac Stimulants Excl. Cardiac Glycosides; Beta2 Agonists; | For inotropic support in the short- term treatment of patients with cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures. |
| FDBD00937 | Primaquine |
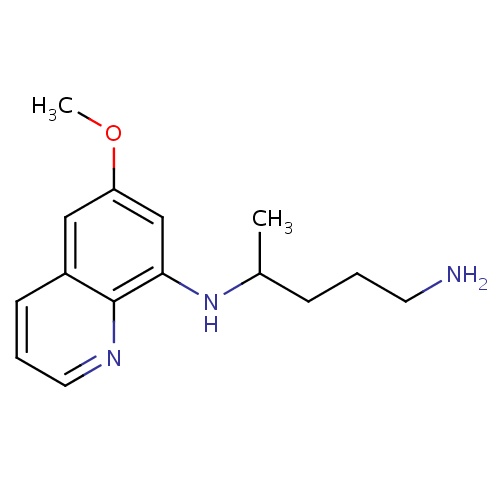
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of malaria. |
| FDBD01261 | Hydroxychloroquine |
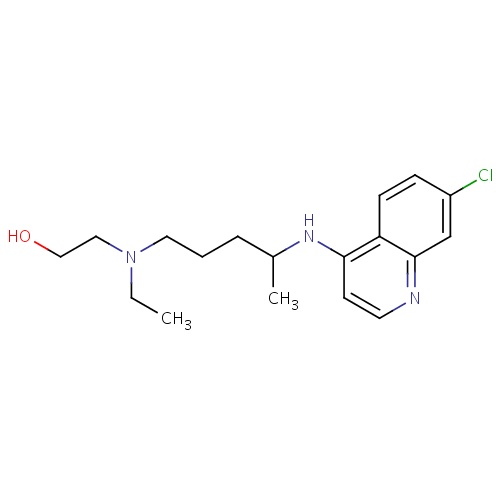
|
Antirheumatic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the suppressive treatment and treatment of acute attacks of malaria due to . |
| FDBD01508 | Viomycin |
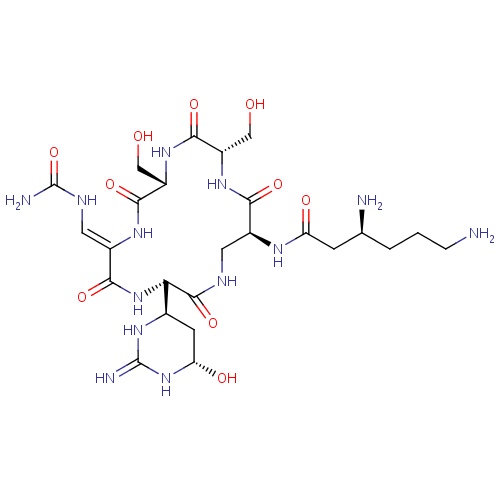
|
Anti-Bacterial Agents; Protein Synthesis Inhibitors; Antibiotics, Antitubercular; | Viomycin is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. |
| FDBD02518 | butralin |
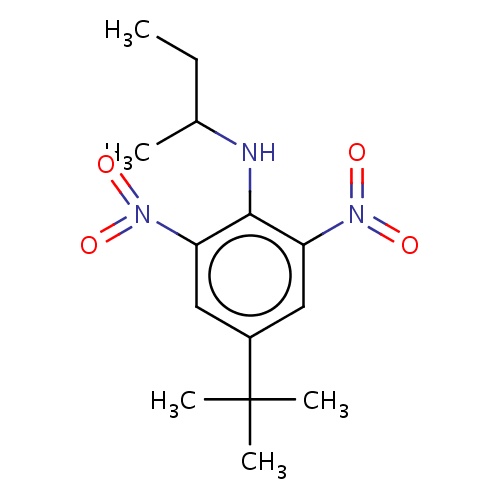
|
Herbicide | Herbicide |
| FDBD02592 | butamifos |

|
Herbicide | Herbicide |
6 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4fmu_ligand_3_21.mol2 | 4fmu | 1 | -6.26 | [C@@H]([NH3+])(C)CC | 5 |
| 2fdp_ligand_3_78.mol2 | 2fdp | 1 | -6.18 | C([C@H](C)[NH3+])C | 5 |
| 2oah_ligand_3_164.mol2 | 2oah | 1 | -6.17 | C([C@H](C)[NH3+])C | 5 |
| 1qaq_ligand_3_10.mol2 | 1qaq | 1 | -6.11 | C[C@H](CC)[NH3+] | 5 |
| 2oah_ligand_3_171.mol2 | 2oah | 1 | -6.10 | C[C@H](CC)[NH3+] | 5 |
| 3cbp_ligand_3_10.mol2 | 3cbp | 1 | -6.04 | C[C@H](CC)[NH3+] | 5 |
| 3bx5_ligand_2_9.mol2 | 3bx5 | 1 | -5.98 | C(C)[C@@H](C)[NH3+] | 5 |
| 2h2j_ligand_3_10.mol2 | 2h2j | 1 | -5.94 | C[C@H](CC)[NH3+] | 5 |
| 1jqe_ligand_3_49.mol2 | 1jqe | 1 | -5.90 | CC[C@@H](C)[NH3+] | 5 |
| 4awo_ligand_2_0.mol2 | 4awo | 1 | -5.86 | C(C)[C@H]([NH3+])C | 5 |
331 ,
34

