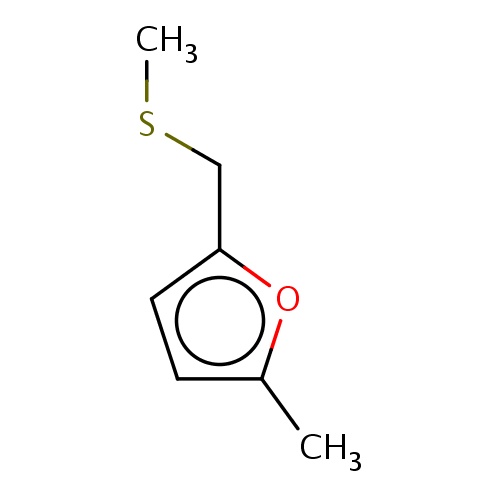
Common name
2-methyl-5-(methylsulfanylmethyl)furan
IUPAC name
2-methyl-5-(methylsulfanylmethyl)furan
SMILES
CSCc1oc(cc1)C
Common name
2-methyl-5-(methylsulfanylmethyl)furan
IUPAC name
2-methyl-5-(methylsulfanylmethyl)furan
SMILES
CSCc1oc(cc1)C
INCHI
InChI=1S/C7H10OS/c1-6-3-4-7(8-6)5-9-2/h3-4H,5H2,1-2H3
FORMULA
C7H10OS

Common name
2-methyl-5-(methylsulfanylmethyl)furan
IUPAC name
2-methyl-5-(methylsulfanylmethyl)furan
Molecular weight
142.219
clogP
2.563
clogS
-2.573
Frequency
0.0003
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
38.44
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00723 | Ranitidine |
|
Anti-Ulcer Agents; Alimentary Tract and Metabolism; Drugs for Peptic Ulcer and Gastro-Oesophageal Reflux Disease (Gord); Drugs for Acid Related Disorders; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; H2 Antagonists; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Used in the treatment of peptic ulcer disease (PUD), dyspepsia, stress ulcer prophylaxis, and gastroesophageal reflux disease (GERD). |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2be2_ligand_3_19.mol2 | 2be2 | 0.930233 | -6.08 | CSCc1occc1 | 8 |
| 2be2_ligand_2_14.mol2 | 2be2 | 0.744186 | -6.12 | C(S)c1occc1 | 7 |
| 2hwh_ligand_1_0.mol2 | 2hwh | 0.511628 | -5.68 | Cc1ccc(o1)C | 7 |
| 2uy5_ligand_1_0.mol2 | 2uy5 | 0.488372 | -6.43 | Cc1ccco1 | 6 |
| 4bo9_ligand_1_4.mol2 | 4bo9 | 0.488372 | -6.22 | Cc1occc1 | 6 |
| 4oc3_ligand_1_9.mol2 | 4oc3 | 0.488372 | -6.14 | c1(ccco1)C | 6 |
| 2xn5_ligand_1_3.mol2 | 2xn5 | 0.488372 | -6.13 | Cc1ccco1 | 6 |
102 ,
11

