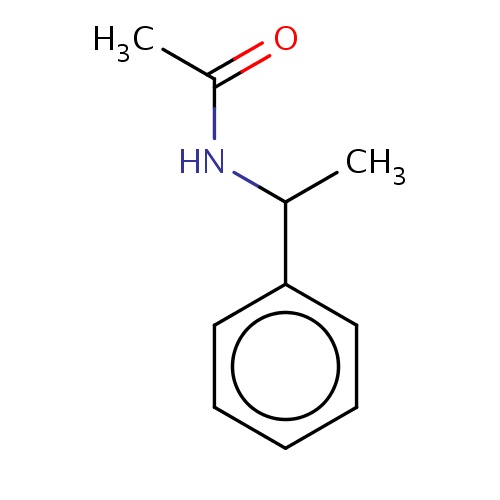
Common name
N-[(1S)-1-phenylethyl]acetamide
IUPAC name
N-[(1S)-1-phenylethyl]acetamide
SMILES
CC(=O)NC(c1ccccc1)C
Common name
N-[(1S)-1-phenylethyl]acetamide
IUPAC name
N-[(1S)-1-phenylethyl]acetamide
SMILES
CC(=O)NC(c1ccccc1)C
INCHI
InChI=1S/C10H13NO/c1-8(11-9(2)12)10-6-4-3-5-7-10/h3-8H,1-2H3,(H,11,12)/t8-/m0/s1
FORMULA
C10H13NO

Common name
N-[(1S)-1-phenylethyl]acetamide
IUPAC name
N-[(1S)-1-phenylethyl]acetamide
Molecular weight
163.216
clogP
1.889
clogS
-2.454
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00769 | Repaglinide |
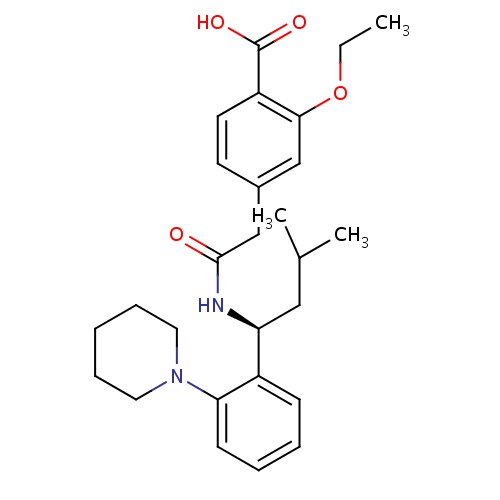
|
Hypoglycemic Agents; Antidiabetic Agents; Meglitinides; Drugs Used in Diabetes; Alimentary Tract and Metabolism; Blood Glucose Lowering Drugs, Excl. Insulins; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4l7o_ligand_3_10.mol2 | 4l7o | 1 | -6.79 | CC(=O)N[C@@H](C)c1ccccc1 | 12 |
| 4l7n_ligand_3_10.mol2 | 4l7n | 1 | -6.78 | CC(=O)N[C@@H](C)c1ccccc1 | 12 |
| 1bil_ligand_4_11.mol2 | 1bil | 1 | -6.70 | CC(=O)N(C)[C@H](c1ccccc1)C | 13 |
| 1bil_ligand_3_25.mol2 | 1bil | 1 | -6.63 | CC(=O)N[C@H](c1ccccc1)C | 12 |
| 1bim_ligand_3_0.mol2 | 1bim | 1 | -6.59 | CC(=O)N([C@H](c1ccccc1)C)C | 13 |
| 4gid_ligand_2_90.mol2 | 4gid | 0.894737 | -6.82 | c1(ccccc1)[C@H](NC=O)C | 11 |
| 4s1g_ligand_2_14.mol2 | 4s1g | 0.894737 | -6.82 | c1(ccccc1)[C@H](C)NC=O | 11 |
| 3ixj_ligand_2_18.mol2 | 3ixj | 0.894737 | -6.72 | c1(ccccc1)[C@H](NC=O)C | 11 |
| 3ixk_ligand_2_18.mol2 | 3ixk | 0.894737 | -6.67 | c1(ccccc1)[C@@H](C)NC=O | 11 |
104 ,
11

