
Common name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
IUPAC name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
SMILES
O=C1C(C(C(C1)O)C=CCC(O)C)C
Common name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
IUPAC name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
SMILES
O=C1C(C(C(C1)O)C=CCC(O)C)C
INCHI
InChI=1S/C11H18O3/c1-7(12)4-3-5-9-8(2)10(13)6-11(9)14/h3,5,7-9,11-12,14H,4,6H2,1-2H3/b5-3+/t7-,8-,9-,11-/m1/s1
FORMULA
C11H18O3

Common name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
IUPAC name
(2R,3R,4R)-4-hydroxy-3-[(E,4R)-4-hydroxypent-1-enyl]-2-methyl-cyclopentanone
Molecular weight
198.259
clogP
1.422
clogS
-0.638
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
57.53
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00785 | Misoprostol |
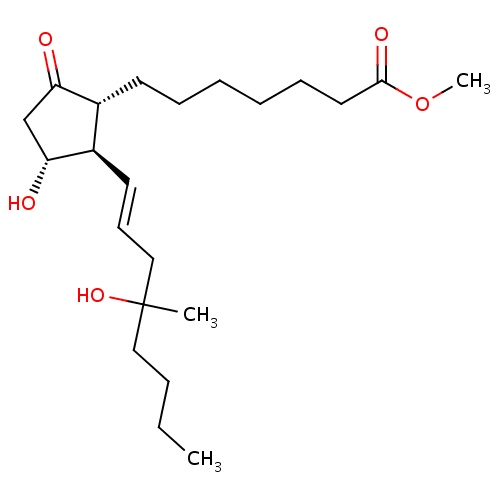
|
Indicated for the treatment of ulceration (duodenal, gastric and NSAID induced) and prophylaxis for NSAID induced ulceration. |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1gni_ligand.mol2 | 1gni | 0.75 | -8.55 | C(=O)(O)CCCCCCC/C=C\CCCCCCCC | 21 |
| 1hms_ligand.mol2 | 1hms | 0.75 | -8.09 | C(C(=O)O)CCCCCC/C=C\CCCCCCCC | 21 |
| 1lfo_ligand.mol2 | 1lfo | 0.75 | -8.08 | C(=O)(O)CCCCCCC/C=C\CCCCCCCC | 21 |
| 4tkj_ligand.mol2 | 4tkj | 0.75 | -7.99 | C(=O)(O)CCCCCCCCCCC/C=C/CC | 19 |
| 1g74_ligand.mol2 | 1g74 | 0.75 | -7.96 | C(=O)(O)CCCCCCC/C=C\CCCCCCCC | 21 |
| 4lkt_ligand.mol2 | 4lkt | 0.75 | -7.93 | C(=O)(CCCCCCCCCC/C=C\CCCCC)O | 21 |
| 1vyf_ligand.mol2 | 1vyf | 0.75 | -7.90 | C(=O)(O)CCCCCCC/C=C\CCCCCCCC | 21 |
| 4tkh_ligand.mol2 | 4tkh | 0.75 | -7.63 | C(=O)(O)CCCCCCCCC/C=C/CC | 17 |
| 2lkk_ligand.mol2 | 2lkk | 0.75 | -7.59 | C(=O)(O)CCCCCCC/C=C\CCCCCCCC | 21 |

