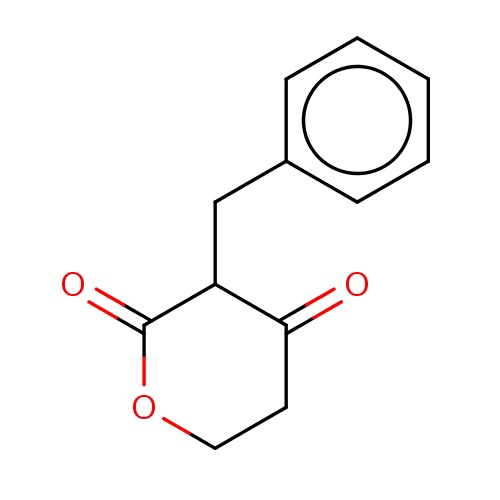
Common name
(3R)-3-benzyltetrahydropyran-2,4-dione
IUPAC name
(3R)-3-benzyltetrahydropyran-2,4-dione
SMILES
C(C1C(=O)CCOC1=O)c2ccccc2
Common name
(3R)-3-benzyltetrahydropyran-2,4-dione
IUPAC name
(3R)-3-benzyltetrahydropyran-2,4-dione
SMILES
C(C1C(=O)CCOC1=O)c2ccccc2
INCHI
InChI=1S/C12H12O3/c13-11-6-7-15-12(14)10(11)8-9-4-2-1-3-5-9/h1-5,10H,6-8H2
FORMULA
C12H12O3

Common name
(3R)-3-benzyltetrahydropyran-2,4-dione
IUPAC name
(3R)-3-benzyltetrahydropyran-2,4-dione
Molecular weight
204.222
clogP
2.725
clogS
-2.726
Frequency
0.0003
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
43.37
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00787 | Tipranavir |
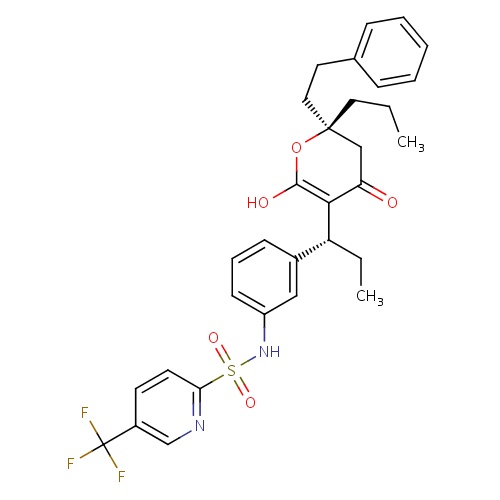
|
Anti-HIV Agents; Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For combination antiretroviral treatment of HIV-1 infected adult patients with evidence of viral replication, who are highly treatment-experienced or have HIV-1 strains resistant to multiple protease inhibitors. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1d4y_ligand_2_5.mol2 | 1d4y | 0.868852 | -7.25 | C([C@@H]1[C@H](CCOC1=O)O)c1ccccc1 | 15 |
| 1d4y_ligand_3_35.mol2 | 1d4y | 0.854839 | -7.82 | [C@H](CC)([C@@H]1[C@H](CCOC1=O)O)c1ccccc1 | 17 |
| 1d4y_ligand_4_54.mol2 | 1d4y | 0.84127 | -8.16 | c1(ccccc1)[C@@H](CC)[C@@H]1[C@H](C[C@@H](OC1=O)C)O | 18 |
| 1d4y_ligand_4_26.mol2 | 1d4y | 0.84127 | -8.15 | c1(ccccc1)[C@@H](CC)[C@@H]1[C@H](C[C@@H](C)OC1=O)O | 18 |
| 1d4y_ligand_4_3.mol2 | 1d4y | 0.84127 | -7.90 | c1(ccccc1)C[C@@H]1[C@H](CC(C)(OC1=O)C)O | 17 |
| 1d4y_ligand_4_16.mol2 | 1d4y | 0.84127 | -7.72 | c1(ccccc1)C[C@@H]1[C@H](C[C@@H](CC)OC1=O)O | 17 |
| 1d4y_ligand_3_12.mol2 | 1d4y | 0.84127 | -7.59 | C([C@@H]1[C@H](C[C@@H](OC1=O)C)O)c1ccccc1 | 16 |
| 1d4y_ligand_3_4.mol2 | 1d4y | 0.84127 | -7.59 | C([C@@H]1[C@H](C[C@H](OC1=O)C)O)c1ccccc1 | 16 |
| 1d4y_ligand_4_15.mol2 | 1d4y | 0.809524 | -7.48 | C[C@@H]1[C@H](C[C@@H](CCc2ccccc2)OC1=O)O | 17 |
| 1d4y_ligand_4_127.mol2 | 1d4y | 0.793651 | -7.51 | C(c1ccccc1)C[C@]1(OC(=O)C[C@H](C1)O)C | 17 |
118 ,
12