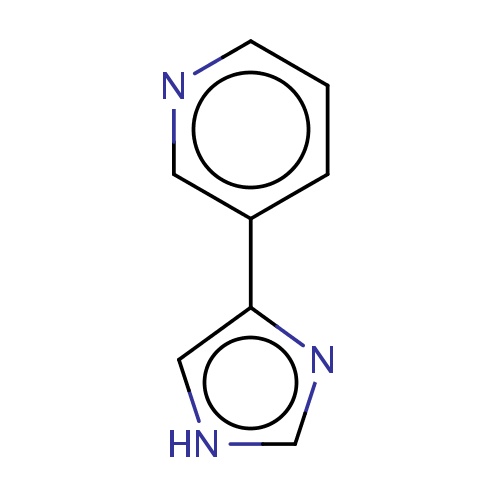
Common name
3-(1H-imidazol-4-yl)pyridine
IUPAC name
3-(1H-imidazol-4-yl)pyridine
SMILES
[nH]1cc(nc1)c2cnccc2
Common name
3-(1H-imidazol-4-yl)pyridine
IUPAC name
3-(1H-imidazol-4-yl)pyridine
SMILES
[nH]1cc(nc1)c2cnccc2
INCHI
InChI=1S/C8H7N3/c1-2-7(4-9-3-1)8-5-10-6-11-8/h1-6H,(H,10,11)
FORMULA
C8H7N3

Common name
3-(1H-imidazol-4-yl)pyridine
IUPAC name
3-(1H-imidazol-4-yl)pyridine
Molecular weight
145.161
clogP
2.131
clogS
-2.449
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
41.57
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
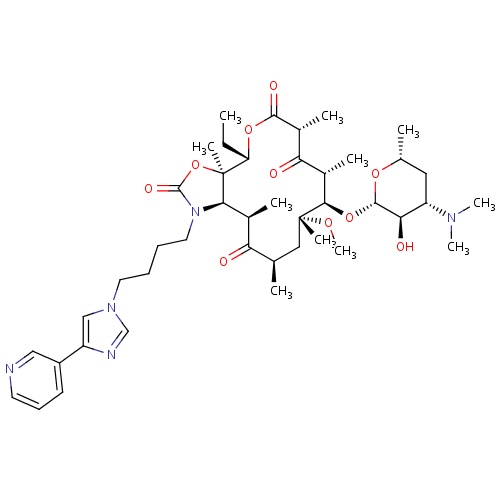
| FDBD00830 | Telithromycin |
|
Anti-Bacterial Agents; Ketolides; Macrolides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of . |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4yl3_ligand_1_2.mol2 | 4yl3 | 0.794872 | -6.37 | c1[nH+]c(c(Br)[nH]1)c1cccnc1 | 12 |
| 1iep_ligand_1_0.mol2 | 1iep | 0.701299 | -7.31 | c1(ccncn1)c1cccnc1 | 12 |
| 3gvu_ligand_1_0.mol2 | 3gvu | 0.701299 | -7.31 | c1(ccncn1)c1cccnc1 | 12 |
| 2hyy_ligand_1_0.mol2 | 2hyy | 0.701299 | -7.29 | c1cc(cnc1)c1ccncn1 | 12 |
| 1t46_ligand_1_0.mol2 | 1t46 | 0.701299 | -7.24 | c1(ccncn1)c1cccnc1 | 12 |
| 4bkj_ligand_1_0.mol2 | 4bkj | 0.701299 | -7.05 | c1(ccncn1)c1cccnc1 | 12 |
| 3be2_ligand_1_9.mol2 | 3be2 | 0.701299 | -6.91 | c1(ccncn1)c1cnccc1 | 12 |
| 3gp0_ligand_1_6.mol2 | 3gp0 | 0.701299 | -6.91 | c1nccc(n1)c1cccnc1 | 12 |
101 ,
11

