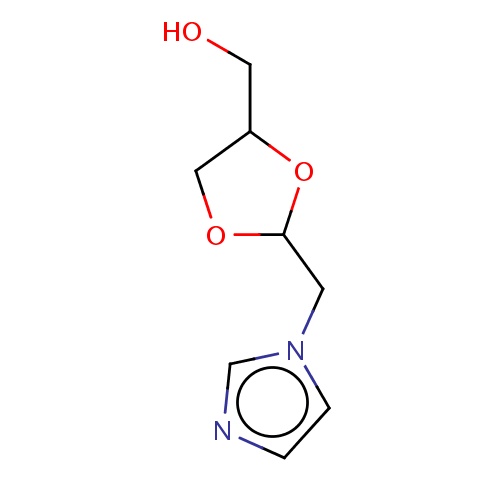
Common name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
IUPAC name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
SMILES
OCC1OC(OC1)Cn2ccnc2
Common name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
IUPAC name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
SMILES
OCC1OC(OC1)Cn2ccnc2
INCHI
InChI=1S/C8H12N2O3/c11-4-7-5-12-8(13-7)3-10-2-1-9-6-10/h1-2,6-8,11H,3-5H2/t7-,8+/m0/s1
FORMULA
C8H12N2O3

Common name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
IUPAC name
[(2R,4S)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methanol
Molecular weight
185.200
clogP
-1.184
clogS
-0.045
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
58.36
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00880 | Ketoconazole |

|
Antifungal Agents; 14-alpha Demethylase Inhibitors; Genito Urinary System and Sex Hormones; Antiinfectives for Systemic Use; Dermatologicals; Gynecological Antiinfectives and Antiseptics; Imidazole and Triazole Derivatives; Antifungals for Topical Use; Antifungals for Dermatological Use; Antimycotics for Systemic Use; Imidazole Derivatives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; BSEP/ABCB11 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of the following systemic fungal infections: candidiasis, chronic mucocutaneous candidiasis, oral thrush, candiduria, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4n1z_ligand_1_7.mol2 | 4n1z | 0.525424 | -5.57 | c1c[nH]c[n+]1CCO | 8 |
| 4ga3_ligand_1_3.mol2 | 4ga3 | 0.525424 | -5.52 | C(O)C[n+]1cc[nH]c1 | 8 |
| 5afx_ligand_1_8.mol2 | 5afx | 0.525424 | -5.48 | [nH]1cc[n+](c1)CCO | 8 |
| 1gym_ligand.mol2 | 1gym | 0.5 | -6.77 | [C@H]1([C@@H]([C@@H]([C@H]([C@@H]([C@H]1O[C@@H]1[C@@H]([C@H]([C@@H]([C@@H](CO)O1)O)O)[NH3+])O)O)O)O)O | 24 |
| 1fkw_ligand.mol2 | 1fkw | 0.462963 | -7.49 | n1cnc2c(c1)ncn2[C@H]1[C@H](O)[C@H](O)[C@H](O1)CO | 19 |
| 1fhd_ligand.mol2 | 1fhd | 0.449612 | -7.97 | [C@@H]1([C@@H]([C@H]([C@@H](CO1)O)O)O)O[C@H]1[C@@H]([C@H](c2n(C1)ccn2)O)O | 22 |
| 1ong_ligand_frag_1.mol2 | 1ong | 0.444444 | -5.55 | c1[nH]c2[n+](c1)CCOC2 | 9 |
102 ,
11

