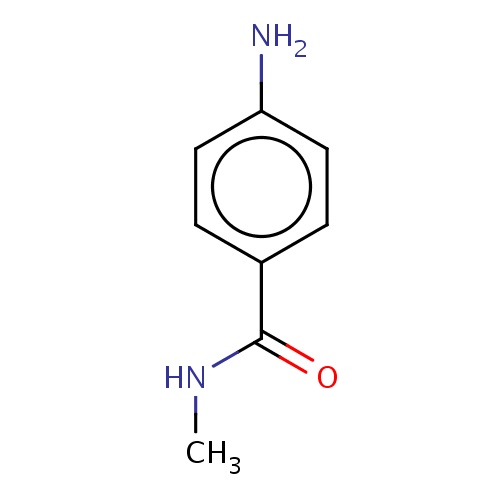
Common name
4-amino-N-methyl-benzamide
IUPAC name
4-amino-N-methyl-benzamide
SMILES
Nc1ccc(cc1)C(=O)NC
Common name
4-amino-N-methyl-benzamide
IUPAC name
4-amino-N-methyl-benzamide
SMILES
Nc1ccc(cc1)C(=O)NC
INCHI
InChI=1S/C8H10N2O/c1-10-8(11)6-2-4-7(9)5-3-6/h2-5H,9H2,1H3,(H,10,11)
FORMULA
C8H10N2O

Common name
4-amino-N-methyl-benzamide
IUPAC name
4-amino-N-methyl-benzamide
Molecular weight
150.178
clogP
0.648
clogS
-1.802
Frequency
0.0007
HBond Acceptor
1
HBond Donor
3
Total PolarSurface Area
55.12
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00888 | Procainamide |
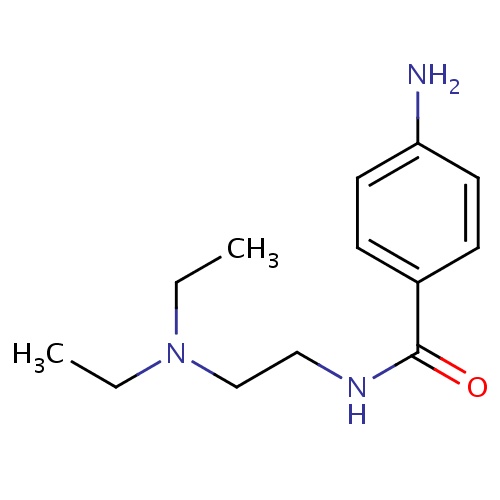
|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ia; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the treatment of life-threatening ventricular arrhythmias. |
| FDBD01405 | Amisulpride |
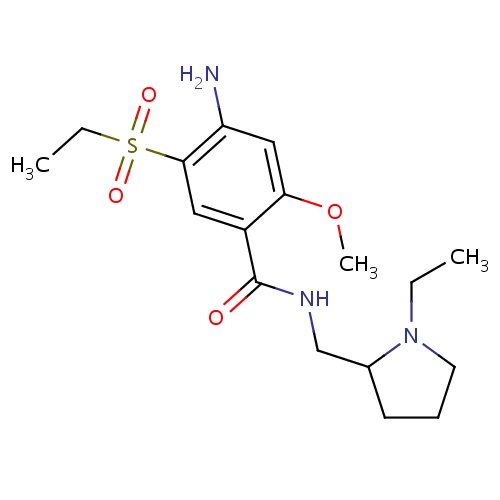
|
Antipsychotic Agents; Dopamine Antagonists; Nervous System; Psycholeptics; Benzamides; | Investigated for use/treatment in schizophrenia and schizoaffective disorders, mania in bipolar disorder, and depression. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1lf2_ligand_2_70.mol2 | 1lf2 | 1 | -6.69 | CNC(=O)c1ccc(cc1)N | 11 |
| 2vgp_ligand_2_2.mol2 | 2vgp | 1 | -6.50 | Nc1ccc(C(=O)NC)cc1 | 11 |
| 4bb4_ligand_3_2.mol2 | 4bb4 | 1 | -6.39 | c1(ccc(cc1)N)C(=O)NC | 11 |
| 1rww_ligand_3_52.mol2 | 1rww | 1 | -6.19 | Nc1ccc(cc1)C(=O)NC | 11 |
| 4r7m_ligand_2_0.mol2 | 4r7m | 1 | -6.06 | C(=O)(NC)c1ccc(cc1)N | 11 |
| 1lf2_ligand_3_268.mol2 | 1lf2 | 0.902439 | -6.95 | C(C)NC(=O)c1ccc(cc1)N | 12 |
| 1lf2_ligand_3_277.mol2 | 1lf2 | 0.902439 | -6.91 | C(C)NC(=O)c1ccc(cc1)N | 12 |
| 4bb4_ligand_4_1.mol2 | 4bb4 | 0.902439 | -6.46 | c1(ccc(cc1)N)C(=O)NCC | 12 |
| 1lf2_ligand_4_701.mol2 | 1lf2 | 0.880952 | -7.26 | C(C)(C)NC(=O)c1ccc(cc1)N | 13 |
| 2oym_ligand_3_0.mol2 | 2oym | 0.880952 | -6.96 | CNC(=O)c1ccc(N(C)C)cc1 | 13 |
200 ,
21

