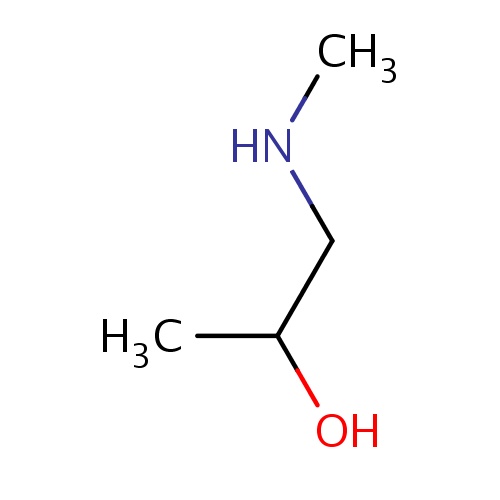
Common name
(2R)-1-(methylamino)propan-2-ol
IUPAC name
(2R)-1-(methylamino)propan-2-ol
SMILES
CNCC(O)C
Common name
(2R)-1-(methylamino)propan-2-ol
IUPAC name
(2R)-1-(methylamino)propan-2-ol
SMILES
CNCC(O)C
INCHI
InChI=1S/C4H11NO/c1-4(6)3-5-2/h4-6H,3H2,1-2H3/t4-/m1/s1
FORMULA
C4H11NO

Common name
(2R)-1-(methylamino)propan-2-ol
IUPAC name
(2R)-1-(methylamino)propan-2-ol
Molecular weight
89.136
clogP
-0.362
clogS
-0.485
Frequency
0.0007
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
32.26
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00923 | Atazanavir |
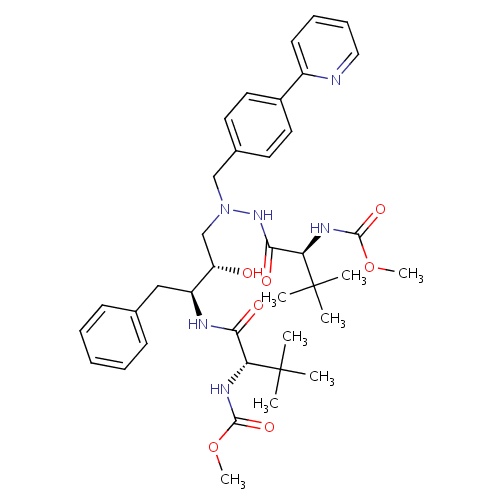
|
Anti-HIV Agents; Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Used in combination with other antiretroviral agents for the treatment of HIV-1 infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. |
| FDBD01674 | Xanthinol |
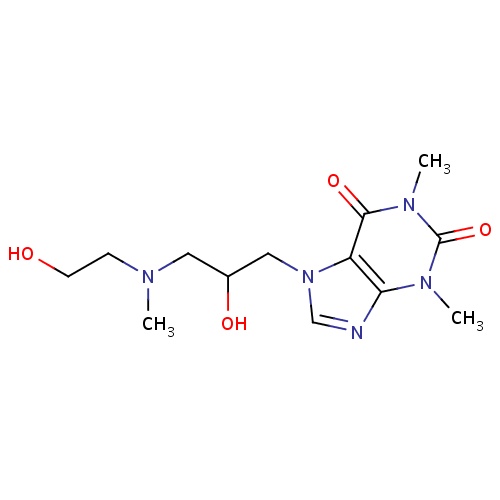
|
Vasodilator Agents; |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vnm_ligand_3_204.mol2 | 2vnm | 1 | -5.95 | C[NH2+]C[C@@H](O)C | 6 |
| 2vnn_ligand_3_104.mol2 | 2vnn | 1 | -5.95 | [C@@H](O)(C[NH2+]C)C | 6 |
| 2wf0_ligand_3_135.mol2 | 2wf0 | 1 | -5.95 | C[C@@H](C[NH2+]C)O | 6 |
| 2vj7_ligand_3_190.mol2 | 2vj7 | 1 | -5.94 | [C@H](O)(C)C[NH2+]C | 6 |
| 2wez_ligand_3_135.mol2 | 2wez | 1 | -5.93 | C[C@H](O)C[NH2+]C | 6 |
| 2wf2_ligand_3_71.mol2 | 2wf2 | 1 | -5.92 | C([NH2+]C)[C@H](C)O | 6 |
| 2wf1_ligand_3_107.mol2 | 2wf1 | 1 | -5.91 | C[C@@H](C[NH2+]C)O | 6 |
| 2ewy_ligand_3_19.mol2 | 2ewy | 1 | -5.90 | C[NH2+]C[C@@H](O)C | 6 |
264 ,
27

