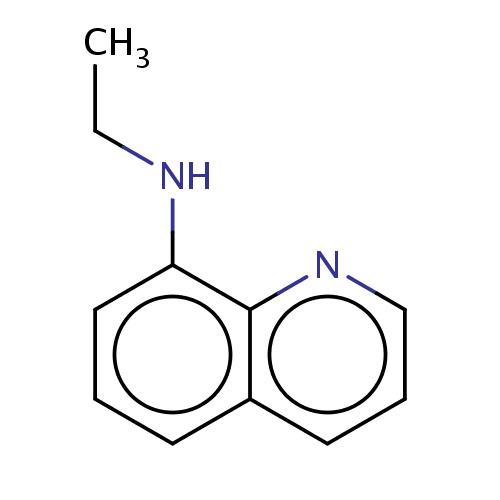
Common name
N-ethylquinolin-8-amine
IUPAC name
N-ethylquinolin-8-amine
SMILES
C(C)Nc1c2ncccc2ccc1
Common name
N-ethylquinolin-8-amine
IUPAC name
N-ethylquinolin-8-amine
SMILES
C(C)Nc1c2ncccc2ccc1
INCHI
InChI=1S/C11H12N2/c1-2-12-10-7-3-5-9-6-4-8-13-11(9)10/h3-8,12H,2H2,1H3
FORMULA
C11H12N2

Common name
N-ethylquinolin-8-amine
IUPAC name
N-ethylquinolin-8-amine
Molecular weight
172.226
clogP
2.463
clogS
-3.631
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
24.92
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00937 | Primaquine |
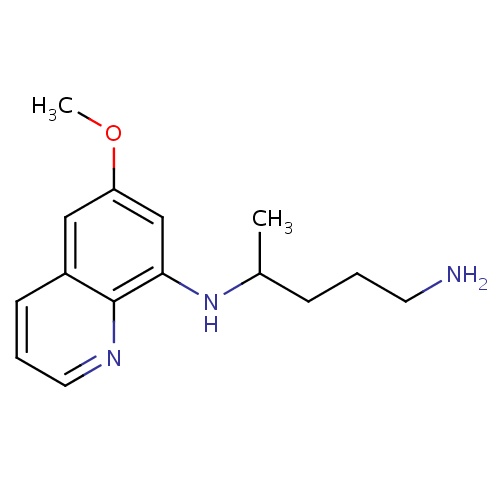
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of malaria. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4yrr_ligand.mol2 | 4yrr | 0.777778 | -6.95 | O=C(C)Nc1cc2c(nc1)cccc2 | 15 |
| 4gg5_ligand_1_0.mol2 | 4gg5 | 0.75 | -7.58 | c1(cc2c(nc1)cccc2)N1CC[NH+](CC1)C | 17 |
| 4mib_ligand_1_3.mol2 | 4mib | 0.75 | -7.18 | c1cccc2c1ncc(c2)N1CCCC1 | 15 |
| 4mib_ligand_2_12.mol2 | 4mib | 0.736842 | -7.40 | c1cccc2c1ncc(c2)N1CC[C@@H](C1)C | 16 |
| 4tkf_ligand_1_2.mol2 | 4tkf | 0.7 | -7.13 | C(=O)Nc1c2ncccc2ccc1 | 13 |
| 4k4f_ligand_1_3.mol2 | 4k4f | 0.7 | -6.96 | c1(cccc2c1nccc2)NC=O | 13 |
| 4yrs_ligand.mol2 | 4yrs | 0.688525 | -6.95 | n1c2ccccc2cc(c1)NC(=O)CC | 16 |
| 4gg5_ligand_3_3.mol2 | 4gg5 | 0.677419 | -7.65 | N(c1c2cc(cnc2ccc1)N1CC[NH+](CC1)C)C | 19 |
| 4gg5_ligand_2_1.mol2 | 4gg5 | 0.677419 | -7.54 | Nc1c2cc(cnc2ccc1)N1CC[NH+](CC1)C | 18 |
| 4jyv_ligand_2_35.mol2 | 4jyv | 0.674419 | -7.08 | CNc1cc2c(cncc2)cc1 | 12 |
207 ,
21

