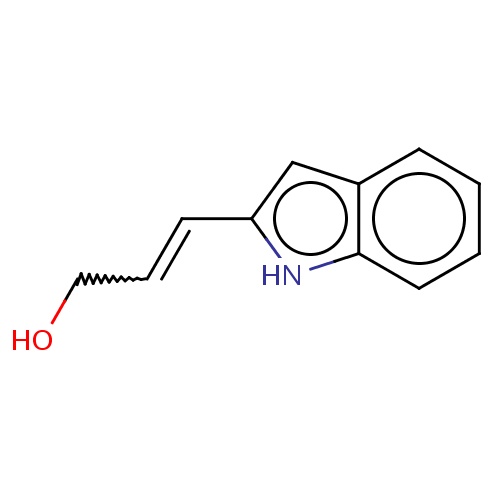
Common name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
IUPAC name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
SMILES
C(O)C=Cc1[nH]c2c(c1)cccc2
Common name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
IUPAC name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
SMILES
C(O)C=Cc1[nH]c2c(c1)cccc2
INCHI
InChI=1S/C11H11NO/c13-7-3-5-10-8-9-4-1-2-6-11(9)12-10/h1-6,8,12-13H,7H2/b5-3+
FORMULA
C11H11NO

Common name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
IUPAC name
(E)-3-(1H-indol-2-yl)prop-2-en-1-ol
Molecular weight
173.211
clogP
2.849
clogS
-2.442
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
36.02
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
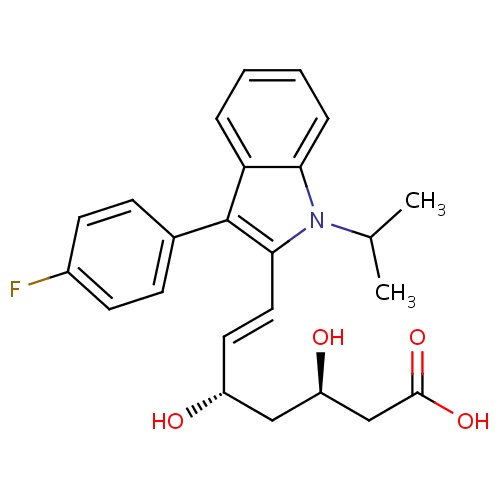
| FDBD00945 | Fluvastatin |
|
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors; HMG CoA Reductase Inhibitors; Lipid Modifying Agents, Plain; Lipid Modifying Agents; Cardiovascular System; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | To be used as an adjunct to dietary therapy to prevent cardiovascular events. May be used as secondary prevention in patients with coronary heart disease (CHD) to reduce the risk of requiring coronary revascularization procedures, for reducing progression of coronary atherosclerosis in hypercholesterolemic patients with CHD, and for the treatment of primary hypercholesterolemia and mixed dyslidipidemia. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4ea2_ligand.mol2 | 4ea2 | 0.590164 | -8.97 | CC(=CCC/C(=C\C[NH2+]CC[NH2+][C@H]1[C@H]2C[C@@H]3C[C@H](C2)C[C@H]1C3)/C)C | 25 |
| 3d50_ligand.mol2 | 3d50 | 0.580247 | -7.29 | [C@H]1([NH2+]CCCCCCCC)[C@H](O)[C@@H](O)[C@H](O)C(=C1)CO | 21 |
| 2zq2_ligand_1_1.mol2 | 2zq2 | 0.574074 | -6.53 | C[NH2+][C@H]1CCCCCC=C1 | 10 |
| 2jf4_ligand.mol2 | 2jf4 | 0.559524 | -9.82 | OCC1=C[C@@H]([C@@H]([C@H]([C@@H]1O)O)O)[NH2+][C@@H]1[C@H](O)[C@H]([C@@H]([C@H](C1)CO)O)O | 24 |
| 2yme_ligand_frag_1.mol2 | 2yme | 0.557143 | -7.04 | C1=C[C@H]2[N@@H+]([C@@H](C1)CCC2)C | 10 |
| 2q7m_ligand_3_36.mol2 | 2q7m | 0.547945 | -6.38 | O=C1C[C@H]2C[C@@H]([N@@H+](C)[C@H]2C=C1)C | 12 |
| 2q7m_ligand_2_12.mol2 | 2q7m | 0.547945 | -6.31 | O=C1C[C@H]2C[C@@H]([NH2+][C@H]2C=C1)C | 11 |
| 4wq3_ligand_2_20.mol2 | 4wq3 | 0.546667 | -7.65 | [C@H]1([C@H]2CC[C@@H](Br)C[C@@H]2[NH2+]C1)/C=C/C(=O)O | 15 |
| 1cj1_ligand_2_63.mol2 | 1cj1 | 0.542857 | -6.69 | [C@H]1(C=CCCC1)NC(=O)C1CCCCC1 | 15 |
| 2q7m_ligand_2_23.mol2 | 2q7m | 0.541667 | -6.22 | O=C1C[C@H]2CC[N@@H+](C)[C@H]2C=C1 | 11 |
522 ,
53

