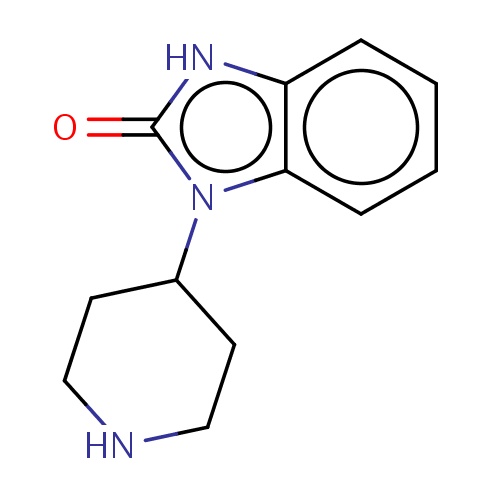
Common name
3-(4-piperidyl)-1H-benzimidazol-2-one
IUPAC name
3-(4-piperidyl)-1H-benzimidazol-2-one
SMILES
O=c1n(c2c([nH]1)cccc2)C3CCNCC3
Common name
3-(4-piperidyl)-1H-benzimidazol-2-one
IUPAC name
3-(4-piperidyl)-1H-benzimidazol-2-one
SMILES
O=c1n(c2c([nH]1)cccc2)C3CCNCC3
INCHI
InChI=1S/C12H15N3O/c16-12-14-10-3-1-2-4-11(10)15(12)9-5-7-13-8-6-9/h1-4,9,13H,5-8H2,(H,14,16)
FORMULA
C12H15N3O

Common name
3-(4-piperidyl)-1H-benzimidazol-2-one
IUPAC name
3-(4-piperidyl)-1H-benzimidazol-2-one
Molecular weight
217.267
clogP
-1.585
clogS
-2.557
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
41.13
Number of Rings
3
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00950 | Pimozide |
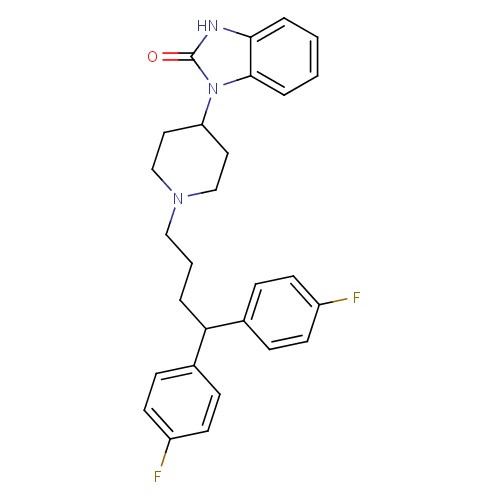
|
Antipsychotic Agents; Dopamine Antagonists; Anti-Dyskinesia Agents; Nervous System; Psycholeptics; Diphenylbutylpiperidine Derivatives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Used for the suppression of motor and phonic tics in patients with Tourette's Disorder who have failed to respond satisfactorily to standard treatment. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2zmm_ligand_2_3.mol2 | 2zmm | 0.611765 | -7.05 | C(=O)(NC)N(c1ccccc1)C1CCCCC1 | 17 |
| 4ocz_ligand.mol2 | 4ocz | 0.604167 | -9.61 | FC(F)(F)c1ccc(cc1)NC(=O)NC1CCN(CC1)C(=O)C(C)C | 26 |
| 4zom_ligand_2_7.mol2 | 4zom | 0.597561 | -7.68 | N(C=O)(C)c1ccccc1N1C[C@@H]2C[C@@H]2C1 | 16 |
| 3mzc_ligand_2_2.mol2 | 3mzc | 0.597561 | -7.01 | c1ccc(cc1)NC(=O)NC1CCCC1 | 15 |
| 1z5m_ligand_1_3.mol2 | 1z5m | 0.592593 | -6.77 | N(C(=O)N1CCCC1)c1ccccc1 | 14 |
| 5ci7_ligand_1_2.mol2 | 5ci7 | 0.592593 | -6.46 | c1cccc(c1)NC(=O)N1CCCC1 | 14 |
101 ,
11

