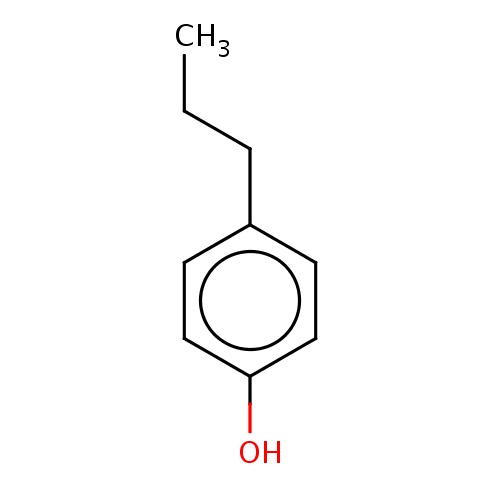
Common name
4-propylphenol
IUPAC name
4-propylphenol
SMILES
C(C)Cc1ccc(cc1)O
Common name
4-propylphenol
IUPAC name
4-propylphenol
SMILES
C(C)Cc1ccc(cc1)O
INCHI
InChI=1S/C9H12O/c1-2-3-8-4-6-9(10)7-5-8/h4-7,10H,2-3H2,1H3
FORMULA
C9H12O

Common name
4-propylphenol
IUPAC name
4-propylphenol
Molecular weight
136.191
clogP
2.483
clogS
-2.382
Frequency
0.0014
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
20.23
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00952 | Arbutamine |
|
Cardiovascular System; Cardiac Therapy; Adrenergic and Dopaminergic Agents; Cardiac Stimulants Excl. Cardiac Glycosides; Beta2 Agonists; | Used to elicit acute cardiovascular responses (cardiac stumulant), similar to those produced by exercise, in order to aid in diagnosing the presence or absence of coronary artery disease (CAD) in patients who cannot exercise adequately. |
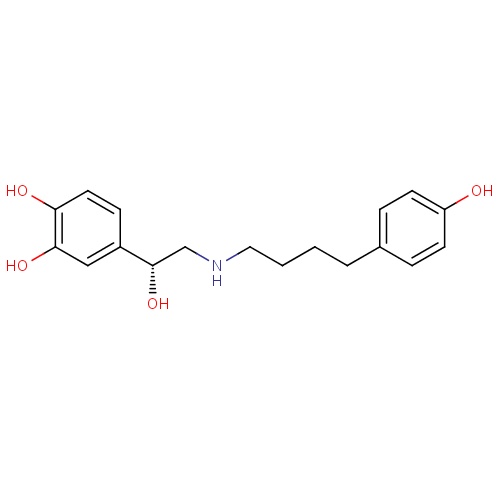
| FDBD01116 | Fenoterol |

|
Sympathomimetics; Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Tocolytic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Genito Urinary System and Sex Hormones; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Adrenergics for Systemic Use; Sympathomimetics, Labour Repressants; Beta2 Agonists; | Fenoterol is used for the treatment of asthma. |
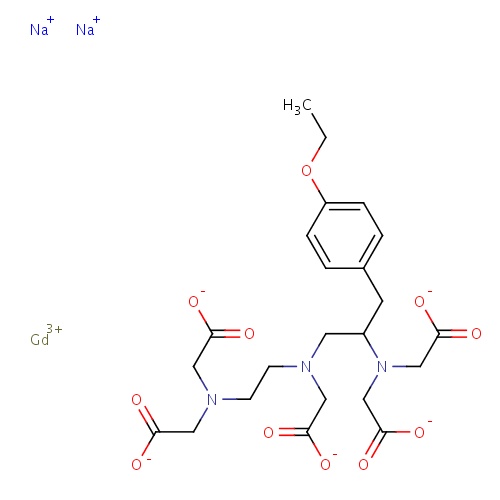
| FDBD01555 | Gadoxetate |
|
Contrast Media; Diagnostic Agents; Paramagnetic Contrast Media; Magnetic Resonance Imaging Contrast Media; | Gadoxetate is used as a contrast medium for magnetic resonance imaging (MRI) to detect and characterize lesions in the liver. |
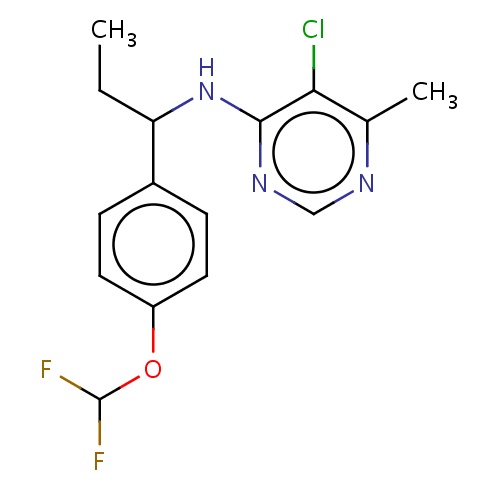
| FDBD03197 | diflumetorim |
|
Fungicide | Fungicide |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1j1a_ligand_4_1330.mol2 | 1j1a | 1 | -6.53 | C(CC)c1ccc(O)cc1 | 10 |
| 1jyq_ligand_3_630.mol2 | 1jyq | 1 | -6.50 | Oc1ccc(cc1)CCC | 10 |
| 1kkq_ligand_4_506.mol2 | 1kkq | 1 | -6.50 | CCCc1ccc(cc1)O | 10 |
| 3mxr_ligand_3_37.mol2 | 3mxr | 1 | -5.63 | CCCc1ccc(cc1)O | 10 |
166 ,
17

