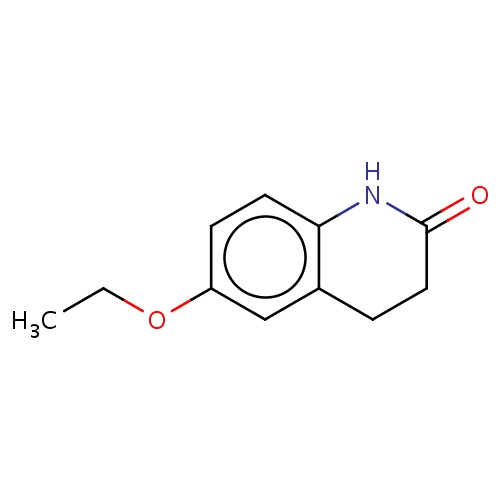
Common name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
IUPAC name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
SMILES
O(c1cc2c(cc1)NC(=O)CC2)CC
Common name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
IUPAC name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
SMILES
O(c1cc2c(cc1)NC(=O)CC2)CC
INCHI
InChI=1S/C11H13NO2/c1-2-14-9-4-5-10-8(7-9)3-6-11(13)12-10/h4-5,7H,2-3,6H2,1H3,(H,12,13)
FORMULA
C11H13NO2

Common name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
IUPAC name
6-ethoxy-3,4-dihydro-1H-quinolin-2-one
Molecular weight
191.226
clogP
2.401
clogS
-3.249
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
38.33
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01012 | Cilostazol |
|
Fibrinolytic Agents; Platelet Aggregation Inhibitors; Bronchodilator Agents; Phosphodiesterase 3 Inhibitors; Vasodilator Agents; Neuroprotective Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the reduction of symptoms of intermittent claudication (pain in the legs that occurs with walking and disappears with rest). |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4pni_ligand_2_18.mol2 | 4pni | 0.875 | -7.55 | CC(=O)N1c2c(cc(OC)cc2)C(CC1)(C)C | 17 |
| 4pni_ligand_1_4.mol2 | 4pni | 0.873786 | -7.44 | O(C)c1ccc2c(c1)C(CCN2C=O)(C)C | 16 |
| 4pni_ligand_3_31.mol2 | 4pni | 0.80531 | -7.61 | Nc1c(OC)cc2c(c1)N(C(=O)C)CCC2(C)C | 18 |
| 4pni_ligand_2_15.mol2 | 4pni | 0.803571 | -7.50 | Nc1c(OC)cc2c(c1)N(C=O)CCC2(C)C | 17 |
| 1ove_ligand_frag_1.mol2 | 1ove | 0.77551 | -6.99 | N1C(=O)CCc2c1cccc2 | 11 |
| 4la7_ligand_frag_0.mol2 | 4la7 | 0.77551 | -6.83 | c12c(cccc1)CCC(=O)N2 | 11 |
| 4n3r_ligand_frag_3.mol2 | 4n3r | 0.767677 | -8.34 | c1cc2c(cc1)C(CC(=O)N2)(C)C | 13 |
104 ,
11

