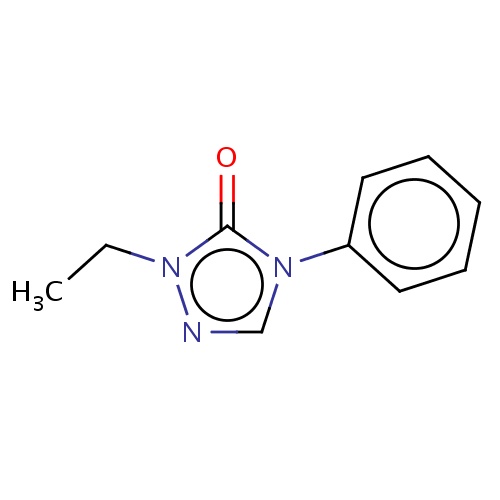
Common name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
IUPAC name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
SMILES
O=c1n(cnn1CC)c2ccccc2
Common name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
IUPAC name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
SMILES
O=c1n(cnn1CC)c2ccccc2
INCHI
InChI=1S/C10H11N3O/c1-2-13-10(14)12(8-11-13)9-6-4-3-5-7-9/h3-8H,2H2,1H3
FORMULA
C10H11N3O

Common name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
IUPAC name
2-ethyl-4-phenyl-1,2,4-triazol-3-one
Molecular weight
191.230
clogP
-2.293
clogS
-1.599
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
32.34
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01013 | Itraconazole |

|
Antifungal Agents; Antiprotozoal Agents; 14-alpha Demethylase Inhibitors; Antiinfectives for Systemic Use; Triazole Derivatives; Antimycotics for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4mw5_ligand_3_34.mol2 | 4mw5 | 0.612903 | -7.37 | C(C)NC(=O)Nc1ccccc1 | 12 |
| 4mvx_ligand_3_12.mol2 | 4mvx | 0.612903 | -7.35 | CCNC(=O)Nc1ccccc1 | 12 |
| 4ekg_ligand_3_28.mol2 | 4ekg | 0.612903 | -7.24 | N(C(=O)NCC)c1ccccc1 | 12 |
| 4eki_ligand_3_36.mol2 | 4eki | 0.612903 | -7.20 | N(C(=O)NCC)c1ccccc1 | 12 |
| 4wvl_ligand_3_14.mol2 | 4wvl | 0.612903 | -6.94 | O=C(Nc1ccccc1)NCC | 12 |
| 4mx1_ligand_3_9.mol2 | 4mx1 | 0.612903 | -6.70 | O=C(NCC)Nc1ccccc1 | 12 |
| 4ngs_ligand_3_7.mol2 | 4ngs | 0.612903 | -6.65 | O=C(NCC)Nc1ccccc1 | 12 |
100 ,
11

