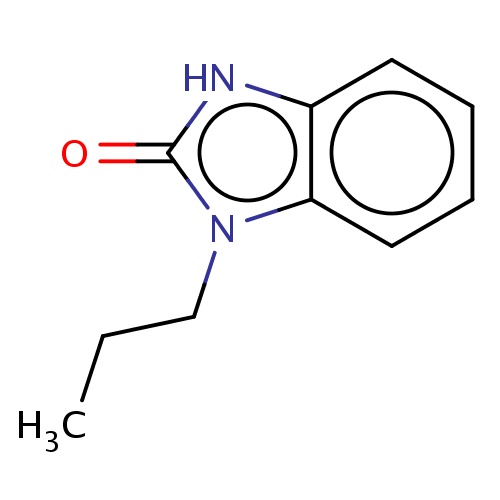
Common name
3-propyl-1H-benzimidazol-2-one
IUPAC name
3-propyl-1H-benzimidazol-2-one
SMILES
O=c1n(c2c([nH]1)cccc2)CCC
Common name
3-propyl-1H-benzimidazol-2-one
IUPAC name
3-propyl-1H-benzimidazol-2-one
SMILES
O=c1n(c2c([nH]1)cccc2)CCC
INCHI
InChI=1S/C10H12N2O/c1-2-7-12-9-6-4-3-5-8(9)11-10(12)13/h3-6H,2,7H2,1H3,(H,11,13)
FORMULA
C10H12N2O

Common name
3-propyl-1H-benzimidazol-2-one
IUPAC name
3-propyl-1H-benzimidazol-2-one
Molecular weight
176.215
clogP
-1.202
clogS
-2.534
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
29.1
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01030 | Domperidone |
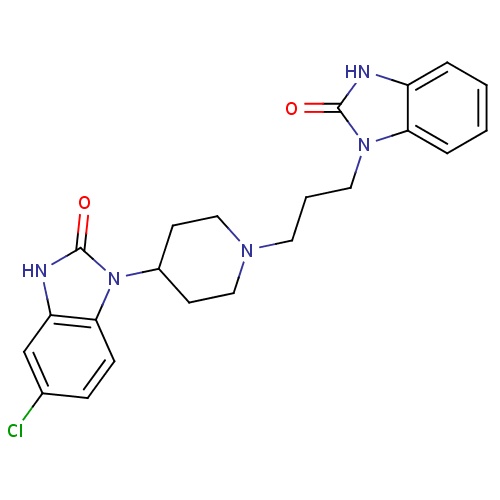
|
Antiprotozoal Agents; Antiemetics; Dopamine Antagonists; Alimentary Tract and Metabolism; Antiparasitic Products, Insecticides and Repellents; Agents Against Protozoal Diseases; Drugs for Functional Gastrointestinal Disorders; Propulsives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For management of dyspepsia, heartburn, epigastric pain, nausea, and vomiting. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4mw5_ligand_4_34.mol2 | 4mw5 | 0.698413 | -7.50 | CCCNC(=O)Nc1ccccc1 | 13 |
| 4ekg_ligand_4_56.mol2 | 4ekg | 0.698413 | -7.33 | N(C(=O)NCCC)c1ccccc1 | 13 |
| 4eki_ligand_4_84.mol2 | 4eki | 0.698413 | -7.28 | N(C(=O)NCCC)c1ccccc1 | 13 |
| 4wvl_ligand_4_91.mol2 | 4wvl | 0.698413 | -6.95 | O=C(Nc1ccccc1)NCCC | 13 |
| 4ngs_ligand_4_6.mol2 | 4ngs | 0.698413 | -6.79 | O=C(NCCC)Nc1ccccc1 | 13 |
| 3hnb_ligand_3_52.mol2 | 3hnb | 0.692308 | -5.98 | N1([C@H](N(c2c1cccc2)CC)[NH3+])C | 13 |
| 4pmp_ligand_3_33.mol2 | 4pmp | 0.6875 | -7.72 | CN(C(=O)Nc1ccccc1)C1CC1 | 14 |
| 4pmp_ligand_2_16.mol2 | 4pmp | 0.6875 | -7.60 | O=C(Nc1ccccc1)NC1CC1 | 13 |
131 ,
14

