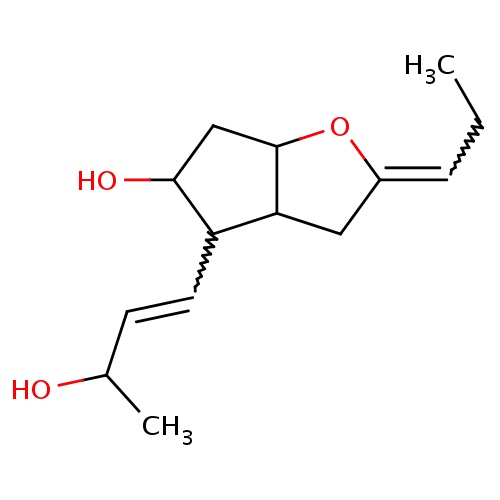
Common name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
IUPAC name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
SMILES
C(=CC(O)C)C1C2C(OC(=CCC)C2)CC1O
Common name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
IUPAC name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
SMILES
C(=CC(O)C)C1C2C(OC(=CCC)C2)CC1O
INCHI
InChI=1S/C14H22O3/c1-3-4-10-7-12-11(6-5-9(2)15)13(16)8-14(12)17-10/h4-6,9,11-16H,3,7-8H2,1-2H3/b6-5+,10-4-/t9-,11+,12+,13+,14-/m0/s1
FORMULA
C14H22O3

Common name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
IUPAC name
(2Z,3aR,4R,5R,6aS)-4-[(E,3S)-3-hydroxybut-1-enyl]-2-propylidene-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-5-ol
Molecular weight
238.323
clogP
2.026
clogS
-0.842
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
49.69
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01084 | Epoprostenol |
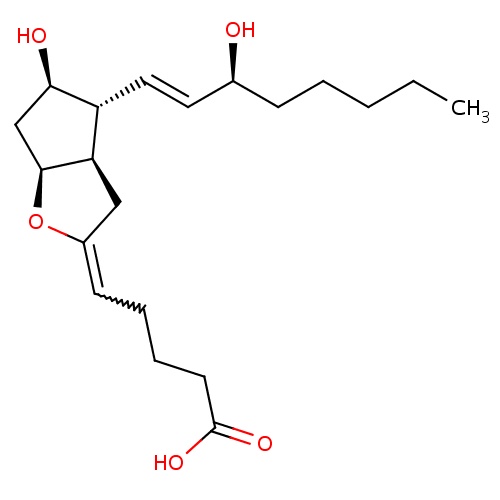
|
Platelet Aggregation Inhibitors; Antihypertensive Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; | For the long-term intravenous treatment of primary pulmonary hypertension and pulmonary hypertension associated with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy. |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1zky_ligand_1_0.mol2 | 1zky | 0.586667 | -7.58 | C(O)[C@@]12[C@H](C)[C@@H](C(=C[C@H]1C)C)COC2 | 14 |
| 2fai_ligand_1_0.mol2 | 2fai | 0.586667 | -7.30 | C[C@@H]1[C@H]2C(=CC[C@@]1(COC2)CO)C | 13 |
| 2b1v_ligand_1_0.mol2 | 2b1v | 0.586667 | -7.07 | C(O)[C@@]12C[C@@H](C(=CC1)C)COC2 | 12 |
| 3e6y_ligand_2_35.mol2 | 3e6y | 0.585714 | -7.77 | O(C)C[C@@]1(C2=C[C@@]3([C@@H](C[C@@H]([C@@H]([C@@H]2CC1)C)O)CCC3)C)O | 21 |
| 1n0s_ligand_frag_0.mol2 | 1n0s | 0.552083 | -8.08 | C1(=O)C=C2[OH+][C@H]3C[C@@H](CC[C@@H]3C[C@@H]2C=C1)O | 16 |
| 2f14_ligand_frag_6.mol2 | 2f14 | 0.552083 | -5.89 | C1[C@H]2C=CC(=O)C=C2[OH+][C@@H]2C[C@@H](CC[C@@H]12)O | 16 |
| 4tw8_ligand_1_0.mol2 | 4tw8 | 0.55 | -5.43 | C[C@H]1[C@H]2[OH+]C3=CC(=O)C=C[C@@H]3C[C@@H]2CC=C1O | 17 |
| 4tw8_ligand_frag_0.mol2 | 4tw8 | 0.55 | -5.28 | C1[C@H]2C=CC(=O)C=C2[OH+][C@H]2[C@H]1CC=C(C2)O | 16 |
| 1o9e_ligand_2_17.mol2 | 1o9e | 0.544118 | -7.71 | O(C)C[C@@H]1C2=C[C@@]3([C@@H](CC[C@@H]3O)C[C@@H]([C@@H]([C@@H]2CC1)C)O)C | 21 |

