
Common name
N-ethyl-N-methyl-aniline
IUPAC name
N-ethyl-N-methyl-aniline
SMILES
C(C)N(c1ccccc1)C
Common name
N-ethyl-N-methyl-aniline
IUPAC name
N-ethyl-N-methyl-aniline
SMILES
C(C)N(c1ccccc1)C
INCHI
InChI=1S/C9H13N/c1-3-10(2)9-7-5-4-6-8-9/h4-8H,3H2,1-2H3
FORMULA
C9H13N

Common name
N-ethyl-N-methyl-aniline
IUPAC name
N-ethyl-N-methyl-aniline
Molecular weight
135.206
clogP
1.736
clogS
-2.124
Frequency
0.0014
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01088 | Bepridil |
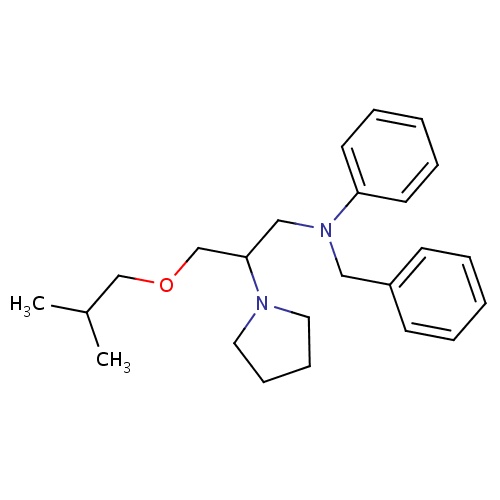
|
Antihypertensive Agents; Anti-Arrhythmia Agents; Vasodilator Agents; Calcium Channel Blockers; Cardiovascular System; Phenylalkylamine Derivatives; Non-Selective Calcium Channel Blockers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the treatment of hypertension, and chronic stable angina (classic effort-associated angina). |
| FDBD01759 | Efonidipine |
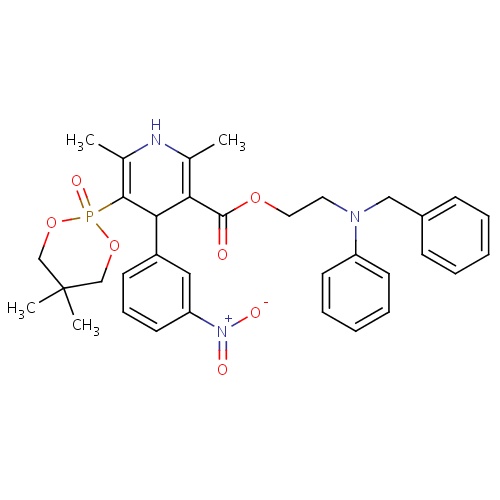
|
; | For the treatment of hypertension. |
| FDBD01810 | Osimertinib |
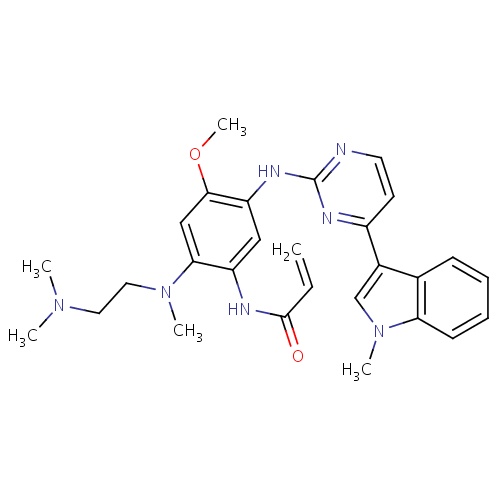
|
Antineoplastic Agents; Protein Kinase Inhibitors; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP3A4 Inhibitors; | Osimertinib is indicated for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA- approved test, who have progressed on or after EGFR-TKI therapy. |
| FDBD02441 | cambendichlor |
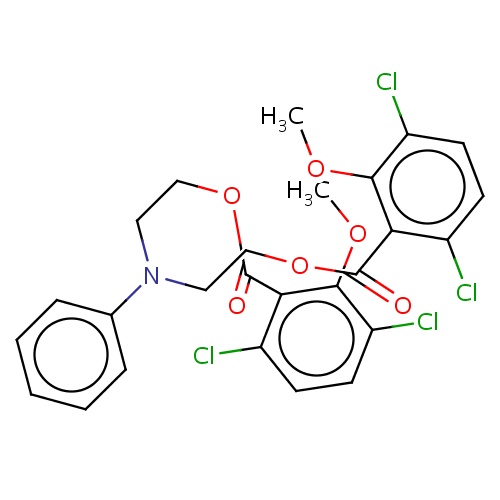
|
Herbicide | Herbicide |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1lxf_ligand_4_159.mol2 | 1lxf | 1 | -6.84 | CCN(c1ccccc1)C | 10 |
| 1lxf_ligand_3_72.mol2 | 1lxf | 1 | -6.75 | CCNc1ccccc1 | 9 |
| 4hj2_ligand_4_2310.mol2 | 4hj2 | 1 | -6.68 | CN(CC)c1ccccc1 | 10 |
| 4hj2_ligand_3_625.mol2 | 4hj2 | 1 | -6.62 | CCNc1ccccc1 | 9 |
| 3d0b_ligand_3_10.mol2 | 3d0b | 1 | -6.59 | C(Nc1ccccc1)C | 9 |
| 2g2r_ligand_3_25.mol2 | 2g2r | 1 | -6.53 | C(C)N(c1ccccc1)C | 10 |
| 4q6e_ligand_3_6.mol2 | 4q6e | 1 | -6.46 | C(Nc1ccccc1)C | 9 |
| 2vj9_ligand_2_7.mol2 | 2vj9 | 1 | -6.39 | C(C)Nc1ccccc1 | 9 |
157 ,
16

