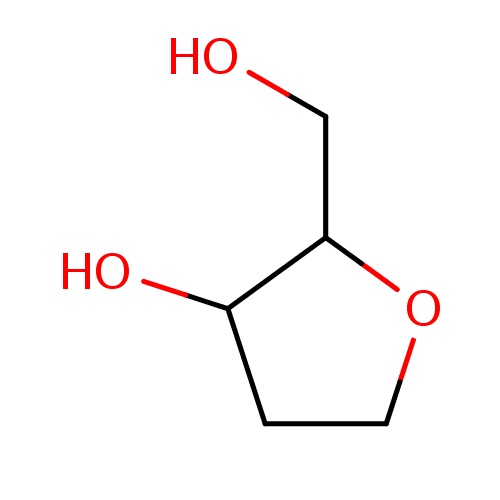
Common name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
SMILES
O1CCC(C1CO)O
Common name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
SMILES
O1CCC(C1CO)O
INCHI
InChI=1S/C5H10O3/c6-3-5-4(7)1-2-8-5/h4-7H,1-3H2/t4-,5+/m1/s1
FORMULA
C5H10O3

Common name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
IUPAC name
(2S,3R)-2-(hydroxymethyl)tetrahydrofuran-3-ol
Molecular weight
118.131
clogP
0.099
clogS
0.528
Frequency
0.0003
HBond Acceptor
3
HBond Donor
2
Total PolarSurface Area
49.69
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01107 | Telbivudine |

|
Antiviral Agents; Nucleic Acid Synthesis Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Nucleoside and Nucleotide Reverse Transcriptase Inhibitors; | For the treatment of chronic hepatitis B in adult and adolescent patients |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2py4_ligand_2_6.mol2 | 2py4 | 1 | -6.37 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 3ehw_ligand_2_6.mol2 | 3ehw | 1 | -6.35 | OC[C@@H]1[C@H](CCO1)O | 8 |
| 2yay_ligand_2_6.mol2 | 2yay | 1 | -6.25 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 2yb0_ligand_1_1.mol2 | 2yb0 | 1 | -6.25 | C1C[C@@H]([C@H](O1)CO)O | 8 |
| 4uxh_ligand_2_15.mol2 | 4uxh | 1 | -6.21 | C([C@H]1OCC[C@@H]1O)O | 8 |
| 2yaz_ligand_2_3.mol2 | 2yaz | 1 | -6.17 | C(O)[C@@H]1[C@H](CCO1)O | 8 |
| 1z34_ligand_1_0.mol2 | 1z34 | 1 | -6.09 | C(O)[C@H]1OCC[C@@H]1O | 8 |
| 3ipx_ligand_1_1.mol2 | 3ipx | 1 | -6.07 | C1O[C@@H]([C@H](C1)O)CO | 8 |
| 4knz_ligand_2_5.mol2 | 4knz | 1 | -6.07 | OC[C@@H]1[C@H](CCO1)O | 8 |
| 2qch_ligand_2_3.mol2 | 2qch | 1 | -6.05 | C(O)[C@@H]1[C@H](CCO1)O | 8 |
171 ,
18

