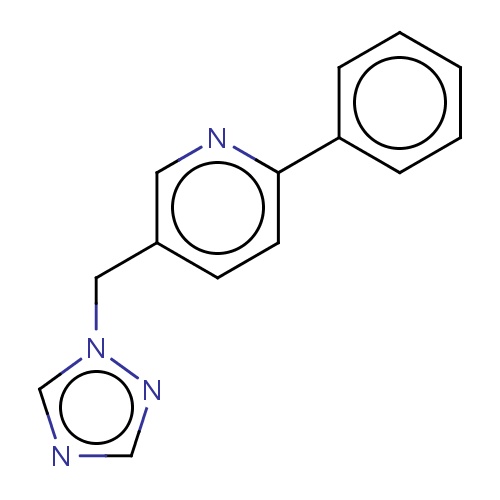
Common name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
IUPAC name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
SMILES
n1(ncnc1)Cc2cnc(cc2)c3ccccc3
Common name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
IUPAC name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
SMILES
n1(ncnc1)Cc2cnc(cc2)c3ccccc3
INCHI
InChI=1S/C14H12N4/c1-2-4-13(5-3-1)14-7-6-12(8-16-14)9-18-11-15-10-17-18/h1-8,10-11H,9H2
FORMULA
C14H12N4

Common name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
IUPAC name
2-phenyl-5-(1,2,4-triazol-1-ylmethyl)pyridine
Molecular weight
237.280
clogP
1.164
clogS
-3.601
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
45.45
Number of Rings
3
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01145 | Forasartan |
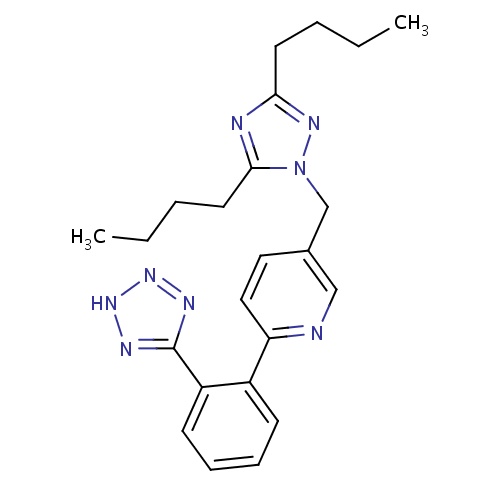
|
Angiotensin Receptor Antagonists; Angiotensin II Receptor Antagonists; | For the treatment of hypertension. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5aix_ligand_2_5.mol2 | 5aix | 0.615385 | -7.96 | c1cccc(c1)c1ccc(C(=O)N)cn1 | 15 |
| 3iph_ligand_2_1.mol2 | 3iph | 0.581818 | -8.33 | c1(ccccc1C)c1ccc(cn1)C(=O)N | 16 |
| 2c5y_ligand_3_0.mol2 | 2c5y | 0.554622 | -7.89 | c1(ncncc1)c1cc(ccc1)C[n+]1c[nH]cn1 | 18 |
| 2c5x_ligand_3_0.mol2 | 2c5x | 0.554622 | -7.75 | c1(cc(ccc1)c1ncncc1)C[n+]1c[nH]cn1 | 18 |
| 3n4c_ligand_1_2.mol2 | 3n4c | 0.53719 | -6.65 | c12c([n+](c[nH]1)C)cc(nc2)c1ccccc1 | 16 |
| 4i8x_ligand.mol2 | 4i8x | 0.537037 | -7.38 | c1(ccccc1)c1ncc(cc1)C(=O)O | 16 |
| 3n4c_ligand_2_7.mol2 | 3n4c | 0.523438 | -6.60 | c12c([n+](c[nH]1)C)cc(nc2)c1ccc(cc1)O | 17 |
| 4ejn_ligand_3_19.mol2 | 4ejn | 0.515152 | -8.78 | Cc1ccc(cc1)[n+]1c2c(ccc(c3ccccc3)n2)[nH]c1 | 22 |
| 3n4c_ligand_2_13.mol2 | 3n4c | 0.514493 | -6.72 | c12c([n+](c[nH]1)C)cc(nc2C=N)c1ccccc1 | 18 |
| 3n4c_ligand_3_22.mol2 | 3n4c | 0.511278 | -6.55 | c12c([n+](c[nH]1)C)cc(nc2)c1ccc(cc1)OC | 18 |
101 ,
11

