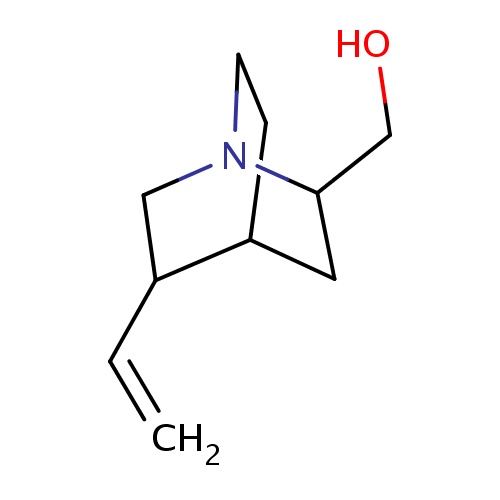
Common name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
IUPAC name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
SMILES
C(O)C1CC2CCN1CC2C=C
Common name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
IUPAC name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
SMILES
C(O)C1CC2CCN1CC2C=C
INCHI
InChI=1S/C10H17NO/c1-2-8-6-11-4-3-9(8)5-10(11)7-12/h2,8-10,12H,1,3-7H2/t8-,9+,10-/m0/s1
FORMULA
C10H17NO

Common name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
IUPAC name
[(2S,4R,5R)-5-vinylquinuclidin-2-yl]methanol
Molecular weight
167.248
clogP
1.222
clogS
-0.732
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
23.47
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01146 | Quinidine barbiturate |

|
; |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4uin_ligand_2_1.mol2 | 4uin | 1 | -6.44 | C(O)[C@H]1[N@H+]2CC[C@H]([C@H](C2)C=C)C1 | 12 |
| 4uil_ligand_2_1.mol2 | 4uil | 1 | -6.19 | C(=C)[C@@H]1[C@H]2CC[N@H+]([C@H](CO)C2)C1 | 12 |
| 4uin_ligand_1_0.mol2 | 4uin | 0.731707 | -6.10 | C(O)[C@H]1[N@H+]2CC[C@H](CC2)C1 | 10 |
| 4uil_ligand_1_0.mol2 | 4uil | 0.731707 | -5.83 | C(O)[C@H]1[N@H+]2CC[C@H](CC2)C1 | 10 |
| 3cib_ligand_4_701.mol2 | 3cib | 0.714286 | -6.93 | C[C@@H]1C[C@@H]([NH2+]CC1)[C@@H](O)CC | 11 |
| 3cib_ligand_3_271.mol2 | 3cib | 0.714286 | -6.79 | C[C@@H]1C[C@@H]([NH2+]CC1)[C@H](C)O | 10 |
| 2qp8_ligand_3_266.mol2 | 2qp8 | 0.714286 | -6.74 | [C@@H]1(CCCC[NH2+]1)[C@H](CC)O | 10 |
| 3cib_ligand_3_266.mol2 | 3cib | 0.714286 | -6.69 | C(C)[C@@H]([C@H]1CCCC[NH2+]1)O | 10 |
| 2qp8_ligand_2_64.mol2 | 2qp8 | 0.714286 | -6.59 | [C@@H]1(CCCC[NH2+]1)[C@H](C)O | 9 |
| 3cib_ligand_2_64.mol2 | 3cib | 0.714286 | -6.55 | [C@@H]1(CCCC[NH2+]1)[C@H](C)O | 9 |
105 ,
11

