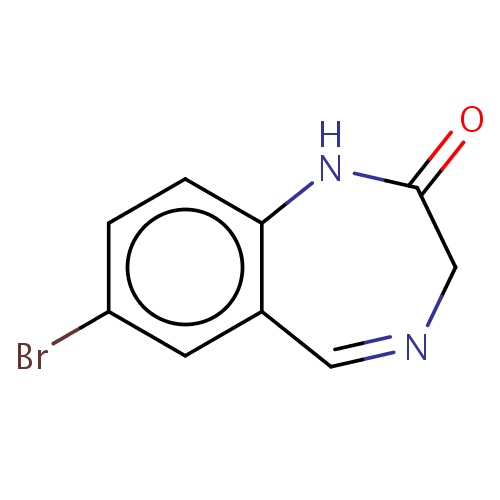
Common name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
IUPAC name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
SMILES
Brc1cc2c(cc1)NC(=O)CN=C2
Common name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
IUPAC name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
SMILES
Brc1cc2c(cc1)NC(=O)CN=C2
INCHI
InChI=1S/C9H7BrN2O/c10-7-1-2-8-6(3-7)4-11-5-9(13)12-8/h1-4H,5H2,(H,12,13)
FORMULA
C9H7BrN2O

Common name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
IUPAC name
7-bromo-1,3-dihydro-1,4-benzodiazepin-2-one
Molecular weight
239.069
clogP
2.755
clogS
-3.387
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
41.46
Number of Rings
2
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
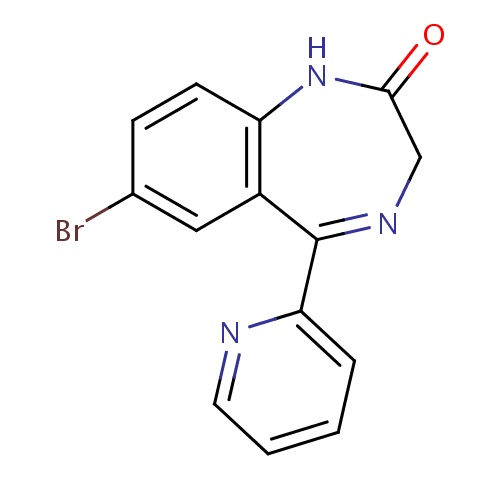
| FDBD01225 | Bromazepam |
|
Anti-Anxiety Agents; GABA Modulators; Benzodiazepines; Nervous System; Benzodiazepine Derivatives; Anxiolytics; Psycholeptics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; | For the short-term treatment of insomnia, short-term treatment of anxiety or panic attacks, if a benzodiazepine is required, and the alleviation of the symptoms of alcohol- and opiate-withdrawal. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1w12_ligand_1_1.mol2 | 1w12 | 0.907563 | -5.71 | C1C(=O)N(c2c(C=N1)cccc2)C | 13 |
| 1w12_ligand_frag_1.mol2 | 1w12 | 0.907563 | -5.62 | C1C(=O)Nc2c(C=N1)cccc2 | 12 |
| 1w12_ligand.mol2 | 1w12 | 0.502283 | -9.03 | N([C@@H]1C(=O)N(CC(=O)N[C@H](C=O)CCC[NH+]=C(N)N)c2c(C(=N1)c1ccccc1)cccc2)S(=O)(=O)Cc1ccccc1 | 44 |
| 3gk2_ligand_2_0.mol2 | 3gk2 | 0.385246 | -6.16 | C1C[NH+](CCN1c1ccccc1C=N)C | 15 |
| 3jup_ligand.mol2 | 3jup | 0.375839 | -8.35 | O=C(O)c1cc(Br)ccc1N[C@H]1CCC[NH2+]C1 | 18 |
| 3juo_ligand.mol2 | 3juo | 0.375839 | -7.89 | O=C(O)c1cc(Br)ccc1N[C@@H]1CCC[NH2+]C1 | 18 |
| 3juq_ligand.mol2 | 3juq | 0.375839 | -7.77 | O=C(O)c1cc(Br)ccc1N[C@@H]1CCC[NH2+]C1 | 18 |
| 4bj9_ligand_2_0.mol2 | 4bj9 | 0.366279 | -7.80 | N(C(=O)C)c1cccc2c1C=NC2=O | 14 |
| 4tk5_ligand_2_0.mol2 | 4tk5 | 0.366279 | -7.75 | c1ccc(c2c1C(=O)N=C2)NC(=O)C | 14 |
| 4rxa_ligand.mol2 | 4rxa | 0.343195 | -9.27 | N1CCN=C1c1cc(ccc1)NC(=O)c1ccc(cc1)c1ccc(cc1)C(=O)Nc1cc(ccc1)C1=NCCN1 | 41 |
103 ,
11

