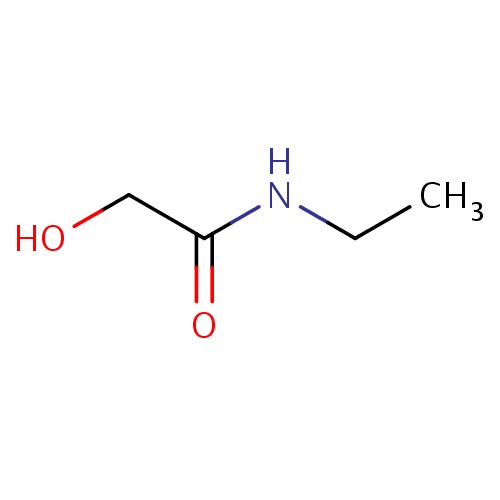
Common name
N-ethyl-2-hydroxy-acetamide
IUPAC name
N-ethyl-2-hydroxy-acetamide
SMILES
C(C(=O)NCC)O
Common name
N-ethyl-2-hydroxy-acetamide
IUPAC name
N-ethyl-2-hydroxy-acetamide
SMILES
C(C(=O)NCC)O
INCHI
InChI=1S/C4H9NO2/c1-2-5-4(7)3-6/h6H,2-3H2,1H3,(H,5,7)
FORMULA
C4H9NO2

Common name
N-ethyl-2-hydroxy-acetamide
IUPAC name
N-ethyl-2-hydroxy-acetamide
Molecular weight
103.120
clogP
-0.495
clogS
-0.428
Frequency
0.0007
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
49.33
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01251 | Lopinavir |
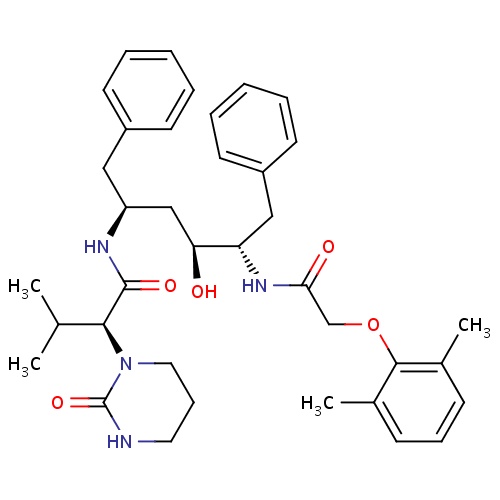
|
Anti-HIV Agents; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Indicated in combination with other antiretroviral agents for the treatment of HIV-infection. |
| FDBD02911 | mandipropamid |
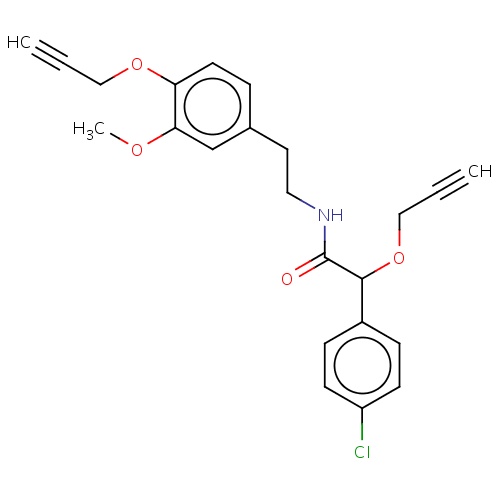
|
Fungicide | Fungicide |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1mj7_ligand_4_220.mol2 | 1mj7 | 1 | -5.86 | N(C(=O)CO)CC | 7 |
| 1tqf_ligand_4_1244.mol2 | 1tqf | 1 | -5.78 | C(=O)(NCC)CO | 7 |
| 1bsk_ligand_4_138.mol2 | 1bsk | 1 | -5.73 | CCNC(=O)CO | 7 |
298 ,
30

