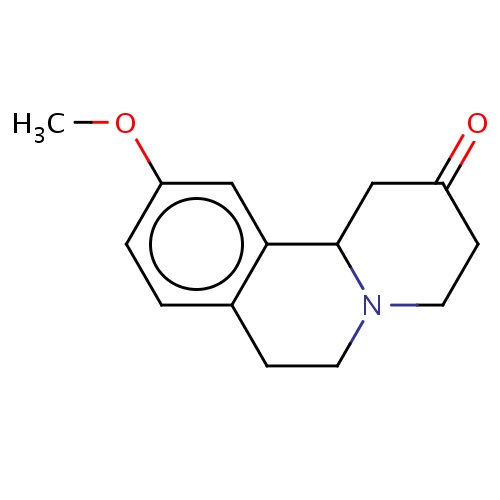
Common name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
IUPAC name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
SMILES
O=C1CCN2C(C1)c3c(ccc(c3)OC)CC2
Common name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
IUPAC name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
SMILES
O=C1CCN2C(C1)c3c(ccc(c3)OC)CC2
INCHI
InChI=1S/C14H17NO2/c1-17-12-3-2-10-4-6-15-7-5-11(16)8-14(15)13(10)9-12/h2-3,9,14H,4-8H2,1H3/t14-/m0/s1
FORMULA
C14H17NO2

Common name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
IUPAC name
(11bS)-10-methoxy-1,3,4,6,7,11b-hexahydropyrido[2,1-a]isoquinolin-2-one
Molecular weight
231.290
clogP
2.461
clogS
-3.012
Frequency
0.0003
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
29.54
Number of Rings
3
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01329 | Tetrabenazine |
|
Adrenergic Uptake Inhibitors; Nervous System; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Treatment of hyperkinetic movement disorders like chorea in Huntington's disease, hemiballismus, senile chorea, Tourette syndrome and other tic disorders, and tardive dyskinesia . |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4djh_ligand.mol2 | 4djh | 0.659091 | -11.01 | C1[NH2+][C@H](Cc2ccc(O)cc12)C(=O)N[C@@H](C(C)C)C[N@H+]1C[C@H](C)[C@](CC1)(C)c1cc(O)ccc1 | 35 |
| 2xyt_ligand.mol2 | 2xyt | 0.633803 | -9.77 | [N@H+]1(CCc2c3[C@@H]1Cc1ccc(Oc4c5[C@@H](Cc6ccc(c(Oc(c(c2)OC)c3)c6)O)[N+](CCc5cc(c4O)OC)(C)C)cc1)C | 46 |
| 4zyi_ligand_2_0.mol2 | 4zyi | 0.632 | -6.36 | C1c2c(cc(c(c2)OC)O)CNC1=O | 14 |
| 4zyf_ligand_2_30.mol2 | 4zyf | 0.632 | -6.25 | Oc1c(OC)cc2c(CNC(=O)C2)c1 | 14 |
| 4zyf_ligand_2_31.mol2 | 4zyf | 0.629032 | -6.92 | C(C)(C)Oc1ccc2c(CNC(=O)C2)c1 | 15 |
| 4zyi_ligand_2_2.mol2 | 4zyi | 0.629032 | -6.48 | C1c2c(cc(cc2)OCC)CNC1=O | 14 |

