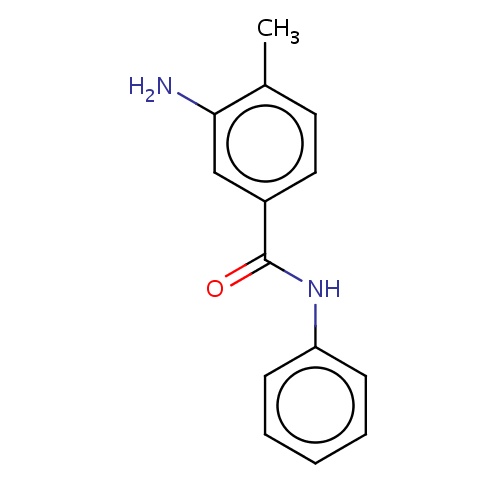
Common name
3-amino-4-methyl-N-phenyl-benzamide
IUPAC name
3-amino-4-methyl-N-phenyl-benzamide
SMILES
c1cc(ccc1)NC(=O)c2cc(c(cc2)C)N
Common name
3-amino-4-methyl-N-phenyl-benzamide
IUPAC name
3-amino-4-methyl-N-phenyl-benzamide
SMILES
c1cc(ccc1)NC(=O)c2cc(c(cc2)C)N
INCHI
InChI=1S/C14H14N2O/c1-10-7-8-11(9-13(10)15)14(17)16-12-5-3-2-4-6-12/h2-9H,15H2,1H3,(H,16,17)
FORMULA
C14H14N2O

Common name
3-amino-4-methyl-N-phenyl-benzamide
IUPAC name
3-amino-4-methyl-N-phenyl-benzamide
Molecular weight
226.274
clogP
2.362
clogS
-3.740
Frequency
0.0003
HBond Acceptor
1
HBond Donor
3
Total PolarSurface Area
55.12
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01336 | Nilotinib |
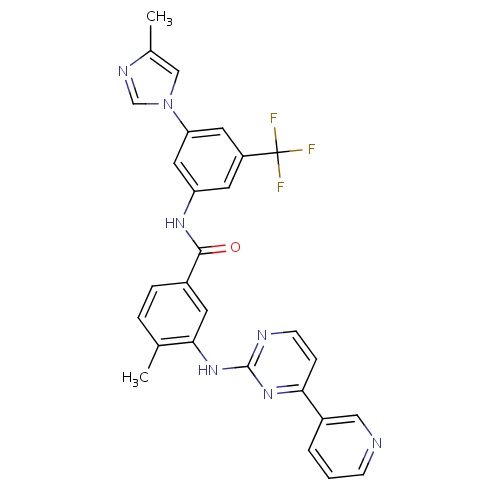
|
Antineoplastic Agents; Immunosuppressive Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the potential treatment of various leukemias, including chronic myeloid leukemia (CML). |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3gp0_ligand_3_2.mol2 | 3gp0 | 1 | -8.75 | O=C(Nc1ccccc1)c1ccc(c(c1)N)C | 17 |
| 2oo8_ligand_3_110.mol2 | 2oo8 | 1 | -8.68 | c1cc(ccc1)NC(=O)c1cc(c(cc1)C)N | 17 |
| 3be2_ligand_3_110.mol2 | 3be2 | 1 | -8.47 | Nc1cc(C(=O)Nc2ccccc2)ccc1C | 17 |
| 2oo8_ligand_4_185.mol2 | 2oo8 | 0.949153 | -8.91 | Nc1cc(C(=O)Nc2cc(ccc2)C)ccc1C | 18 |
| 3be2_ligand_4_185.mol2 | 3be2 | 0.949153 | -8.69 | Nc1cc(C(=O)Nc2cc(C)ccc2)ccc1C | 18 |
| 4x7j_ligand_2_9.mol2 | 4x7j | 0.883333 | -8.58 | c1(ccccc1)NC(=O)c1c(cc(cc1)C)N | 17 |
| 2hz0_ligand_2_0.mol2 | 2hz0 | 0.839286 | -8.73 | C(=O)(Nc1ccccc1)c1ccccc1 | 15 |
| 5ew3_ligand_2_4.mol2 | 5ew3 | 0.839286 | -8.57 | c1cccc(c1)C(=O)Nc1ccccc1 | 15 |
103 ,
11

