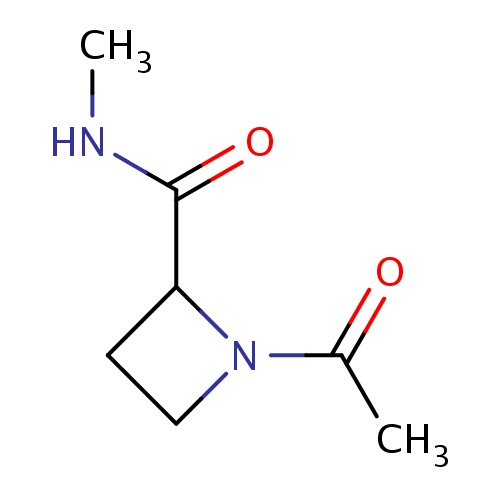
Common name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
IUPAC name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
SMILES
O=C(NC)C1N(CC1)C(=O)C
Common name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
IUPAC name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
SMILES
O=C(NC)C1N(CC1)C(=O)C
INCHI
InChI=1S/C7H12N2O2/c1-5(10)9-4-3-6(9)7(11)8-2/h6H,3-4H2,1-2H3,(H,8,11)/t6-/m0/s1
FORMULA
C7H12N2O2

Common name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
IUPAC name
(2S)-1-acetyl-N-methyl-azetidine-2-carboxamide
Molecular weight
156.182
clogP
-0.205
clogS
-0.525
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
49.41
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01345 | Ximelagatran |
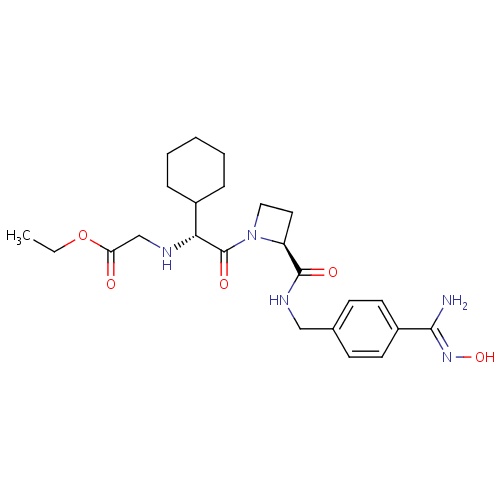
|
Antithrombins; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; | For the treatment of acute deep vein thrombosis. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4bak_ligand_3_80.mol2 | 4bak | 1 | -6.32 | C(=O)(N1CC[C@H]1C(=O)NC)C | 11 |
| 4baq_ligand_2_18.mol2 | 4baq | 0.948718 | -6.16 | C(=O)N1[C@@H](CC1)C(=O)NC | 10 |
| 4ban_ligand_2_15.mol2 | 4ban | 0.948718 | -6.14 | C(=O)(NC)[C@H]1N(C=O)CC1 | 10 |
| 4bao_ligand_2_15.mol2 | 4bao | 0.948718 | -6.13 | C(=O)N1[C@@H](CC1)C(=O)NC | 10 |
| 1k22_ligand_2_9.mol2 | 1k22 | 0.948718 | -6.09 | CNC(=O)[C@H]1N(C=O)CC1 | 10 |
| 4bak_ligand_2_33.mol2 | 4bak | 0.948718 | -6.09 | C(=O)N1CC[C@H]1C(=O)NC | 10 |
| 4bam_ligand_2_15.mol2 | 4bam | 0.948718 | -6.09 | CNC(=O)[C@H]1N(C=O)CC1 | 10 |
| 4bah_ligand_2_12.mol2 | 4bah | 0.948718 | -6.06 | C(=O)(NC)[C@H]1N(C=O)CC1 | 10 |
| 1k1p_ligand_2_12.mol2 | 1k1p | 0.948718 | -5.70 | CNC(=O)[C@H]1N(C=O)CC1 | 10 |
154 ,
16

