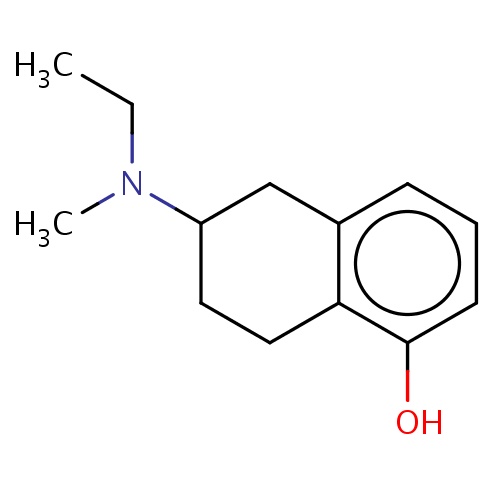
Common name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
IUPAC name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
SMILES
N(CC)(C1CCc2c(cccc2O)C1)C
Common name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
IUPAC name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
SMILES
N(CC)(C1CCc2c(cccc2O)C1)C
INCHI
InChI=1S/C13H19NO/c1-3-14(2)11-7-8-12-10(9-11)5-4-6-13(12)15/h4-6,11,15H,3,7-9H2,1-2H3/t11-/m0/s1
FORMULA
C13H19NO

Common name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
IUPAC name
(2S)-2-[ethyl(methyl)amino]tetralin-5-ol
Molecular weight
205.296
clogP
2.336
clogS
-2.768
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
23.47
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01366 | Rotigotine |
|
Dopamine Agonists; Antidyskinetics; Nervous System; Anti-Parkinson Drugs; Dopaminergic Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For use/treatment in neurologic disorders and parkinson's disease as well as moderate-to-severe primary Restless Legs Syndrome. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2w6c_ligand_3_9.mol2 | 2w6c | 0.689189 | -7.91 | CC[N@H+]1C[C@H](c2cc(ccc2)O)CCCC1 | 16 |
| 2w6c_ligand_2_1.mol2 | 2w6c | 0.689189 | -7.65 | c1(cc(ccc1)[C@H]1C[N@@H+](CCCC1)C)O | 15 |
| 2w6c_ligand_1_0.mol2 | 2w6c | 0.689189 | -7.53 | c1(cc(ccc1)[C@H]1C[NH2+]CCCC1)O | 14 |
| 3h0a_ligand_1_7.mol2 | 3h0a | 0.681159 | -7.23 | c12CCCCc1cccc2O | 11 |
| 2w6c_ligand.mol2 | 2w6c | 0.68 | -9.14 | Oc1cc(ccc1)[C@@]1(CC)C[N@@H+](CCCC1)CCCCCCCCC[NH3+] | 27 |
| 5e8r_ligand_2_13.mol2 | 5e8r | 0.611111 | -6.64 | c1cc(ccc1O)[C@H]1C[NH2+]CC1 | 12 |
| 4djh_ligand_2_14.mol2 | 4djh | 0.60274 | -7.65 | c1(cc(ccc1)O)[C@]1([C@H](C[N@H+](C)CC1)C)C | 16 |
| 5e8r_ligand_3_26.mol2 | 5e8r | 0.60274 | -6.94 | c1cc(ccc1O)[C@H]1C[NH2+]C[C@@H]1C | 13 |
100 ,
11

