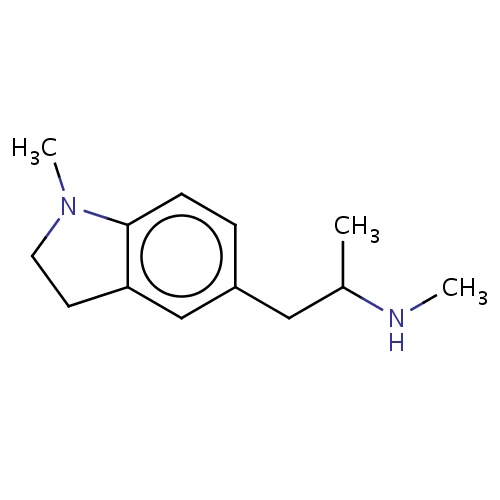
Common name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
IUPAC name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
SMILES
C(C)(Cc1cc2c(cc1)N(CC2)C)NC
Common name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
IUPAC name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
SMILES
C(C)(Cc1cc2c(cc1)N(CC2)C)NC
INCHI
InChI=1S/C13H20N2/c1-10(14-2)8-11-4-5-13-12(9-11)6-7-15(13)3/h4-5,9-10,14H,6-8H2,1-3H3/t10-/m1/s1
FORMULA
C13H20N2

Common name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
IUPAC name
(2R)-N-methyl-1-(1-methylindolin-5-yl)propan-2-amine
Molecular weight
204.311
clogP
2.593
clogS
-3.503
Frequency
0.0003
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
15.27
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01385 | Silodosin |
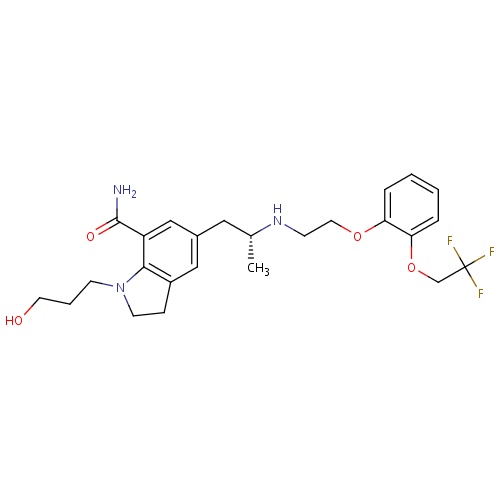
|
Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Urological Agents; CYP3A4 Inhibitors; | Treatment for symptomatic relief of benign prostatic hyperplasia . |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3bl1_ligand_frag_3.mol2 | 3bl1 | 0.826087 | -6.04 | N1[C@H](Cc2c1cccc2)C | 10 |
| 3cp9_ligand_frag_2.mol2 | 3cp9 | 0.820896 | -7.13 | c1ccc2c(c1)NCC2(C)C | 11 |
| 3bkl_ligand_1_0.mol2 | 3bkl | 0.820896 | -6.77 | c12ccccc1NC[C@@H]2C | 10 |
| 1tmn_ligand_1_5.mol2 | 1tmn | 0.820896 | -6.22 | [C@@H]1(CNc2c1cccc2)C | 10 |
| 2ovz_ligand_1_0.mol2 | 2ovz | 0.820896 | -5.86 | C[C@H]1c2ccccc2NC1 | 10 |
| 2ovz_ligand_2_0.mol2 | 2ovz | 0.816901 | -5.93 | C([C@H]1c2ccccc2NC1)C | 11 |
| 1tmn_ligand_frag_10.mol2 | 1tmn | 0.80597 | -5.96 | C1CNc2c1cccc2 | 9 |
| 5ald_ligand.mol2 | 5ald | 0.75 | -8.78 | c1cc2c(cc1C1CCCCC1)CC(=O)N2 | 17 |
100 ,
11

