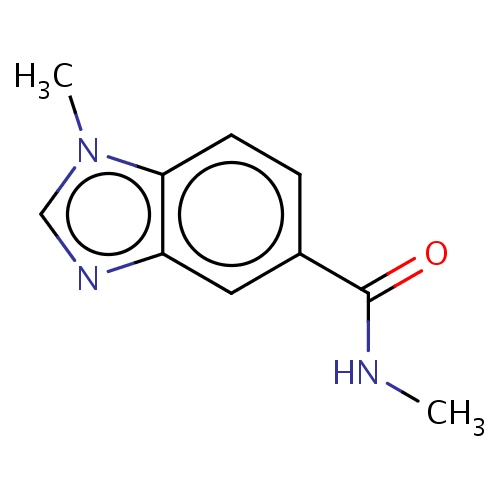
Common name
N,1-dimethylbenzimidazole-5-carboxamide
IUPAC name
N,1-dimethylbenzimidazole-5-carboxamide
SMILES
c1nc2c(n1C)ccc(c2)C(=O)NC
Common name
N,1-dimethylbenzimidazole-5-carboxamide
IUPAC name
N,1-dimethylbenzimidazole-5-carboxamide
SMILES
c1nc2c(n1C)ccc(c2)C(=O)NC
INCHI
InChI=1S/C10H11N3O/c1-11-10(14)7-3-4-9-8(5-7)12-6-13(9)2/h3-6H,1-2H3,(H,11,14)
FORMULA
C10H11N3O

Common name
N,1-dimethylbenzimidazole-5-carboxamide
IUPAC name
N,1-dimethylbenzimidazole-5-carboxamide
Molecular weight
190.222
clogP
-0.206
clogS
-2.261
Frequency
0.0003
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
48.77
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01435 | Dabigatran etexilate |
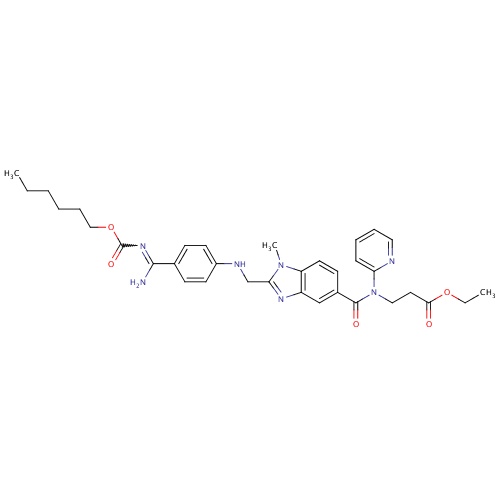
|
Antithrombins; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; | Dabigatran is indicated for the prevention of venous thromboembolic events in patients who have undergone elective hip or knee replacement surgery (based on RE-NOVATE, RE-MODEL, and RE-MOBILIZE trials). In 2010, it was approved in the US and Canada for prevention of stroke and systemic embolism in patients with atrial fibrillation (approval based on the RE-LY trial). Contraindications: severe renal impairment (CrCL . |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4jn2_ligand_2_16.mol2 | 4jn2 | 1 | -6.36 | c1[nH]c2cc(ccc2[n+]1C)C(=O)NC | 14 |
| 4jn2_ligand_3_26.mol2 | 4jn2 | 0.892857 | -6.80 | Cc1[nH]c2cc(ccc2[n+]1C)C(=O)NC | 15 |
| 4uyd_ligand.mol2 | 4uyd | 0.781609 | -6.92 | Cn1c2ccc(cc2n(C)c1=O)C(=O)N | 16 |
| 4jn2_ligand_3_31.mol2 | 4jn2 | 0.735294 | -7.41 | c1[nH]c2cc(ccc2[n+]1C)C(=O)N(C)c1ccccn1 | 20 |
| 5ea5_ligand_3_186.mol2 | 5ea5 | 0.727273 | -6.09 | C(Nc1cc(ccc1)C)c1cc2c([nH]c[n+]2C)cc1 | 19 |
114 ,
12

