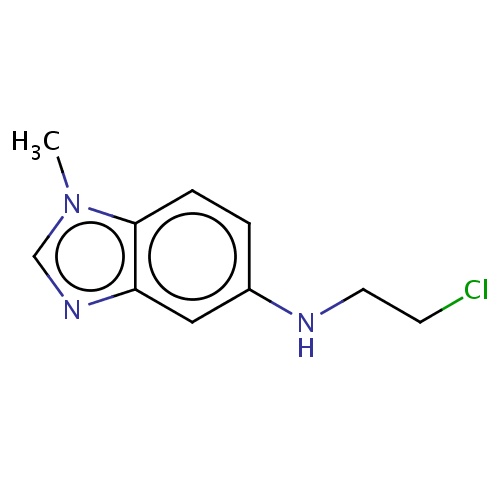
Common name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
IUPAC name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
SMILES
ClCCNc1cc2c(n(cn2)C)cc1
Common name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
IUPAC name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
SMILES
ClCCNc1cc2c(n(cn2)C)cc1
INCHI
InChI=1S/C10H12ClN3/c1-14-7-13-9-6-8(12-5-4-11)2-3-10(9)14/h2-3,6-7,12H,4-5H2,1H3
FORMULA
C10H12ClN3

Common name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
IUPAC name
N-(2-chloroethyl)-1-methyl-benzimidazol-5-amine
Molecular weight
210.683
clogP
0.761
clogS
-3.355
Frequency
0.0003
HBond Acceptor
0
HBond Donor
2
Total PolarSurface Area
31.7
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01471 | Bendamustine |
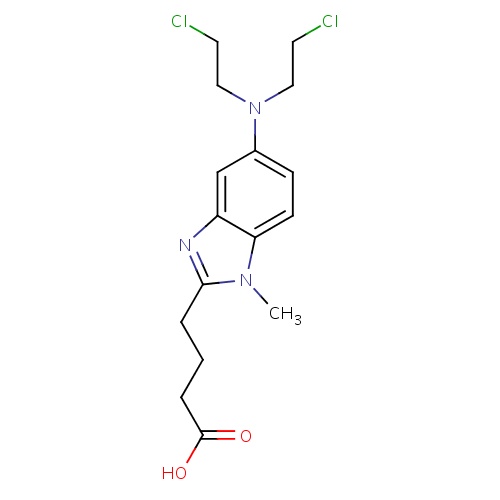
|
Antineoplastic Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrogen Mustard Analogues; | Bendamustine is indicated for treatment of chronic lymphocytic leukemia (CLL). |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4uye_ligand_1_3.mol2 | 4uye | 0.659091 | -7.48 | N1(CCCCC1)c1ccc2n(c(=O)n(c2c1)C)C | 18 |
| 4uye_ligand_1_2.mol2 | 4uye | 0.650602 | -6.95 | c1(cc2n(c(=O)n(c2cc1)C)C)NC=O | 15 |
| 3f8c_ligand_1_4.mol2 | 3f8c | 0.648649 | -6.79 | N1(CC[NH+](CC1)C)c1ccc2c([nH+]c[nH]2)c1 | 16 |
| 5a46_ligand_1_0.mol2 | 5a46 | 0.648649 | -6.16 | C1CN(CC[NH+]1C)c1ccc2[nH+]c[nH]c2c1 | 16 |
| 4qps_ligand_2_0.mol2 | 4qps | 0.644737 | -7.08 | C(C)C(=O)Nc1ccc2c(c1)[nH+]c[nH]2 | 14 |
| 3jzi_ligand_1_1.mol2 | 3jzi | 0.641791 | -6.44 | c1cc(c2[n+](c[nH]c2c1)C)N | 11 |
| 4jvb_ligand_3_110.mol2 | 4jvb | 0.636364 | -7.19 | [n+]1(c2ccccc2[nH]c1)C(C)C | 12 |
| 2xno_ligand_1_2.mol2 | 2xno | 0.621212 | -6.92 | C(C)[n+]1c[nH]c2ccccc12 | 11 |
| 4jvb_ligand_2_36.mol2 | 4jvb | 0.621212 | -6.85 | [n+]1(c2ccccc2[nH]c1)CC | 11 |
101 ,
11

