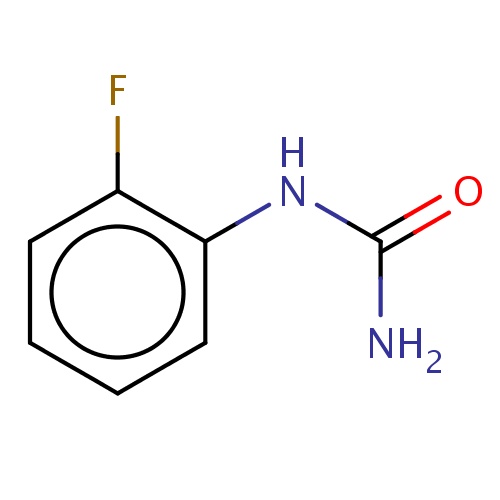
Common name
(2-fluorophenyl)urea
IUPAC name
(2-fluorophenyl)urea
SMILES
Fc1c(cccc1)NC(=O)N
Common name
(2-fluorophenyl)urea
IUPAC name
(2-fluorophenyl)urea
SMILES
Fc1c(cccc1)NC(=O)N
INCHI
InChI=1S/C7H7FN2O/c8-5-3-1-2-4-6(5)10-7(9)11/h1-4H,(H3,9,10,11)
FORMULA
C7H7FN2O

Common name
(2-fluorophenyl)urea
IUPAC name
(2-fluorophenyl)urea
Molecular weight
154.142
clogP
0.620
clogS
-1.697
Frequency
0.0003
HBond Acceptor
1
HBond Donor
3
Total PolarSurface Area
55.12
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01561 | Regorafenib |
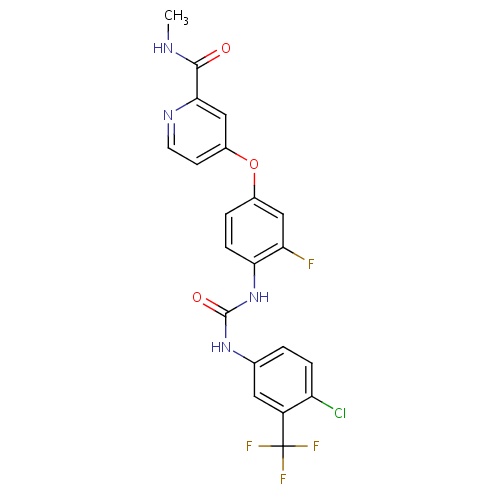
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Regorafenib is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy. Regorafenib is also indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumor (GIST) who have been previously treated with imatinib mesylate and sunitinib malate. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4ksq_ligand_1_3.mol2 | 4ksq | 1 | -7.11 | N(C(=O)N)c1c(F)cccc1 | 11 |
| 4bo8_ligand_1_0.mol2 | 4bo8 | 1 | -7.02 | N(C(=O)N)c1c(F)cccc1 | 11 |
| 2oh4_ligand_1_1.mol2 | 2oh4 | 1 | -6.84 | N(C(=O)N)c1ccccc1F | 11 |
| 4ehg_ligand_1_3.mol2 | 4ehg | 0.95122 | -6.66 | c1(c(cccc1F)F)NC(=O)N | 12 |
155 ,
16

